Group Leader/s
Head of lab
intro
Our laboratory is interested in understanding how cells and its organelles sense and adapt to mechanical signals in physiological and pathological processes.
- Mechanical signals are intrinsically coupled to biochemical reactions. The dynamic behavior of cells is governed by biochemical processes that orchestrate virtually all cellular functions. However, molecular function is modulated not only by biochemical cues but also by mechanical stimuli. Cells, organelles, macromolecular assemblies, and even individual molecules exhibit a high degree of sensitivity to mechanical forces, such as hydrostatic pressure or tensile strain. For instance, mechanical deformation of the nucleus has been shown to rapidly influence gene expression, reflecting the capacity of biomolecules to detect and respond to external mechanical inputs.
- How do cells respond and adapt to mechanical stress? To sense and respond to mechanical stimuli, cells rely on mechanosensitive and mechanoresponsive molecules, some of which have been characterized by our group (Echarri A. et al., Nature Communications, 2019; García-García et al., Nature Communications, 2022; Echarri A., Biomolecules, 2022). Mechanosensitive molecules can undergo physical deformation, such as stretching, which alters their conformation and function—for example, by increasing their affinity for a substrate or exposing novel binding sites for interacting partners. These conformational changes are subsequently translated into biochemical signals in a process known as mechanotransduction, which constitutes a molecular mechanism by which cells interpret and adapt to both external and internal mechanical cues.
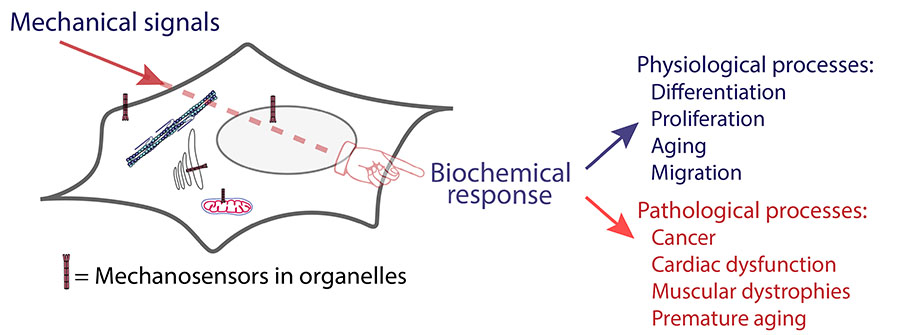
- Human diseases and mechanical stress. Mechanotransduction pathways play a fundamental role in numerous physiological processes, including cell proliferation, differentiation, aging, inflammation, migration, and metabolism. Consequently, mutations in genes encoding components of these pathways are frequently associated with a range of human diseases, such as premature aging syndromes, muscular dystrophies, lipodystrophies, cardiac dysfunction, and cancer (García-García et al., Nature Communications, 2022). Therefore, elucidating the molecular mechanisms underlying the physiological and pathological adaptation of cells to mechanical cues is essential for understanding disease etiology and identifying potential therapeutic targets.
- Our current projects. Despite recent advances, our understanding of mechanotransduction pathways operating within cellular organelles and other subcellular structures remains limited (Biomolecules, 2022, Current Opinion in Cell Biology, 2020). Through integrative bioinformatic analyses, we have identified several candidate pathways potentially involved in cellular mechanotransduction. We are currently studying how the nucleus, specifically the nuclear envelope, adapts to different types of stress, including mechanical stress. In addition, we are investigating the relationship between mechanical stress and the ability of tumor cells to evade the immune response (see specific research lines for further details).
Members
| Asier Echarri Aguirre |
| Carmen Lopez Moran |
| Martin Mauleon Subiza |

Funding
-2023-2026. Project: "Identification and characterization of new mechanotransduction pathways in the mammalian nuclear envelope (MechNuc)"; PID2022-142634NB-I00. Project funded by the Ministry of Science and Innovation, Government of Spain. Program: "Proyectos Generación de Conocimiento".
More info
I am recruiting students for TGF, TFM and FPU applicants. If you are interested in mechanobiology feel free to email me and visit our lab. You can find more information in our lab web page:
https://sites.google.com/view/mechanorganelleslab/inicio