A work recently published in ACS Catalysis by the Structural Biology of Human-Pathogen Interactions led by Dr. Mª Cristina Vega from the Centro de Investigaciones Biológicas Margarita Salas (CSIC), in collaboration with the group of Dr. Iñaki Tuñón (Universitat de València), has unveiled the detailed enzymatic mechanism of a key enzyme for the biosynthesis of the pyrimidine base, essential for the synthesis of nucleic acids.
The enzyme, Orotate phosphoribosyltransferase (OPRTase), catalyzes the reaction between the ribose donor α-D-5-phosphoribosyl-1-pyrophosphate (PRPP) and orotate (OA) in the presence of Mg2+ ion to obtain pyrophosphate and orotidine 5′-monophosphate (OMP). Since OMP is a key precursor in de novo biosynthesis of pyrimidine nucleotides, pharmacological intervention at the level of OPRTase has been shown to compromise the survival of important human pathogens as the malaria parasite (Plasmodium falciparum) and the tuberculosis bacillus (Mycobacterium tuberculosis). Furthermore, OPRTase has been hypothesized to become a relevant target for anti-cancer therapies owing to the elevated supply of pyrimidine nucleotides required to sustain the fast replication rates of neoplastic cells.
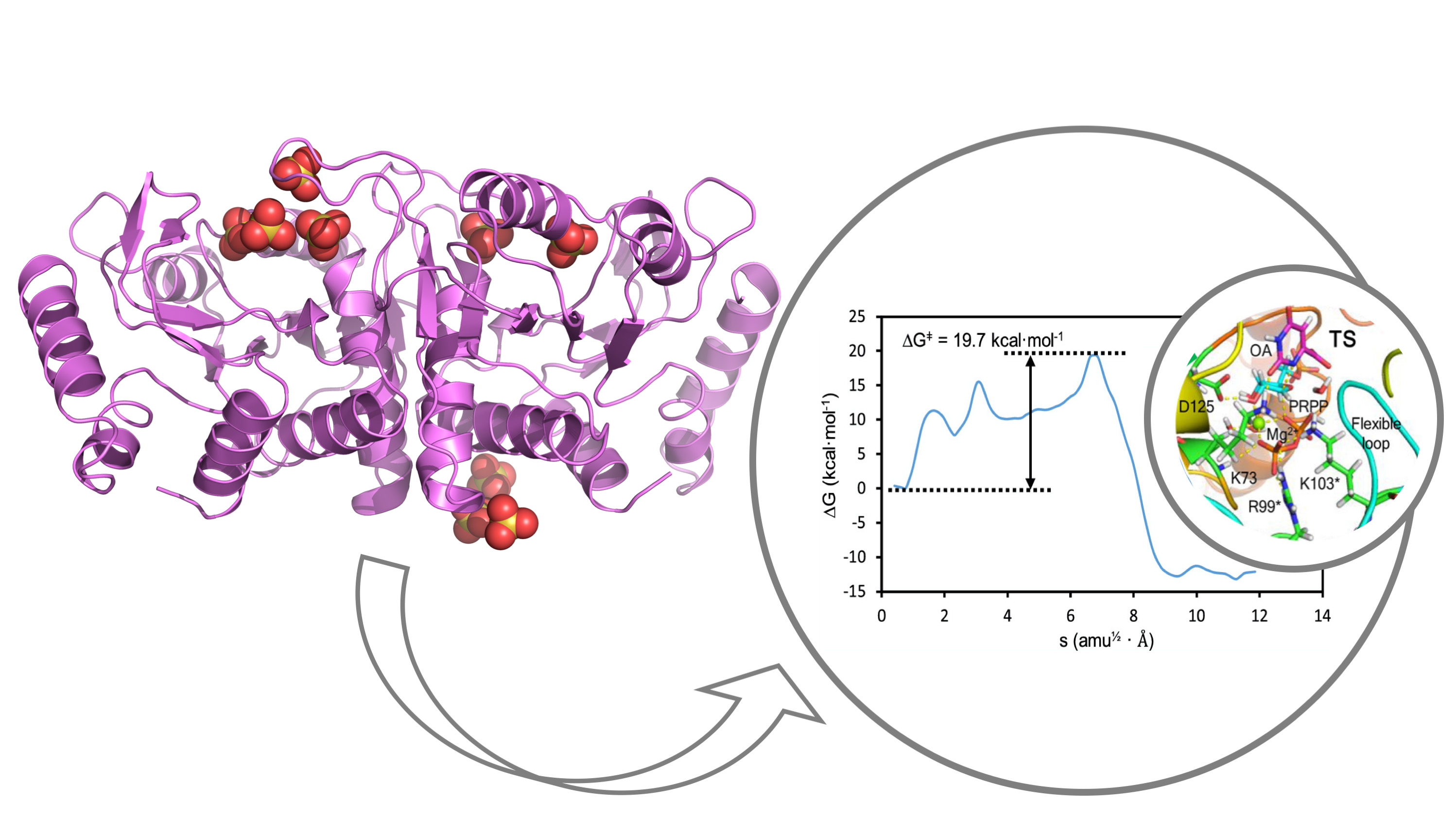
In this work, the Vega group determined the crystallographic structure of various forms of a model enterobacterial OPRTase at high resolution thus generating high-quality models for extensive molecular dynamics and computational chemistry calculations performed by Tuñon group. In addition, the kinetic constants of the enzyme-catalyzed reaction were measured providing an ideal benchmark for comparison with quantum chemical estimates of the height of the activation barrier. The unveiled novel mechanism determines that the enzymatic reaction depends critically on an initial amide conformation for the orotate substrate and a uniquely positioned water molecule. Altogether, the transition state structure for the reaction catalyzed by OPRTase has finally been explained after many decades of discussion. This work paves the way for the discovery of new drugs for the pharmacological inhibition and modulation of the biosynthesis of pyrimidine nucleotides, an essential metabolic pathway.
Reference: “Elucidating the Catalytic Reaction Mechanism of Orotate Phosphoribosyltransferase by Means of X-ray Crystallography and Computational Simulations”. Roca M*, Navas-Yuste S, Zinovjev K, López-Estepa M, Gómez S, Fernández FJ, Vega MC*, Tuñón I* (2020) ACS Catalysis, 10, 1871-1885. DOI:10.1021/acscatal.9b05294

