Pathogenic bacteria from the Firmicutes phylum, such as staphylococcus, streptococcus and enterococcus are the causal agents of several infectious diseases with high rates of morbidity and mortality. In addition, they constitute a reservoir of genetic information that is transferred among them by horizontal gene transfer (conjugation) leading to the spread of antibiotic-resistance traits.
Now, research groups from the Institute of Research in Biomedicine and Centro de Investigaciones Biológicas have solved the three-dimensional structure of the relaxase MobM, protein encoded by the promiscuous plasmid pMV158, which is a key element in the process of horizontal gene transfer.
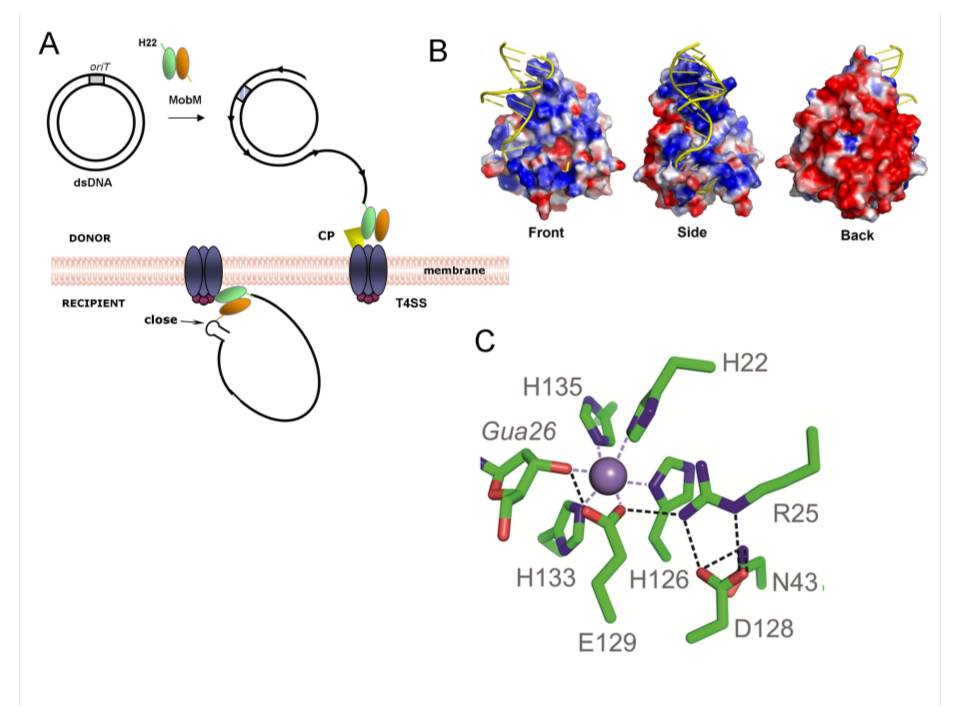
MobM initiates and terminates conjugative plasmid mobilization among bacteria. Conjugative DNA transfer involves physical contact between a donor and a recipient bacterial cell of the same or different species. The transfer is mediated by proteins named relaxases, which recognize and cleave a specific sequence on their DNA target. The MobM-DNA complex is next transferred to the recipient cell by means of a multi-protein complex, the Type IV Secretion System.
The work published in PNAS with the participation of Dr. Manuel Espinosa's group provides the three-dimensional structure of six different MobM-DNA complexes which shows that DNA binds to the protein through regions with electropositive charges. In all the relaxases previously reported, the cleavage reaction was always mediated by a Tyrosine residue. Analyses of the active centre of the protein in the complexes characterize in this study revealed the presence of a Mn2+ ion and, unexpectedly, that the Histidine at position 22 is the responsible to attack the DNA substrate.
These results were confirmed by computational, biochemical, and functional experiments and constitute a novel mechanism for initiation of conjugative transfer and open avenues to explore new tools to reduce the frequency of horizontal gene transfer processes.
Reference: Structural basis of a novel histidine-DNA nicking/joining mechanism for gene transfer and promiscuous spread of antibiotic resistance. Radoslaw Pluta, D. Roeland Boer, Fabián Lorenzo-Díaz, Silvia Russi, Hansel Gómez, Cris Fernández-López, Rosa Pérez-Luque, Modesto Orozco, Manuel Espinosa and Miquel Coll. PNAS (2017). doi: 10.1073/pnas.1702971114.

