Researchers from the Centro de Investigaciones Biológicas Margarita Salas (CIB-CSIC), in collaboration with two groups from the Universitat de València-ERI Biotecmed and CIBERERE-ISCIII, reveal the high-resolution structure of Vip3, in the protoxin and toxin configurations, and shed light on the activation mechanism that triggers cell death. The results of this work, led by Dr. Ernesto Arias, have been published in Nature Communications.
Vip3A is an insecticidal protein secreted by the Bacillus thuringiensis bacteria that has a powerful action against a broad spectrum of Lepidoptera. Today it is used both in organic and conventional crops in spray biopesticides (Bt insecticides), and in transgenic crops protected against insect attack (Bt crops). However, the mechanism by which Vip3A induced its insecticidal action was unknown.
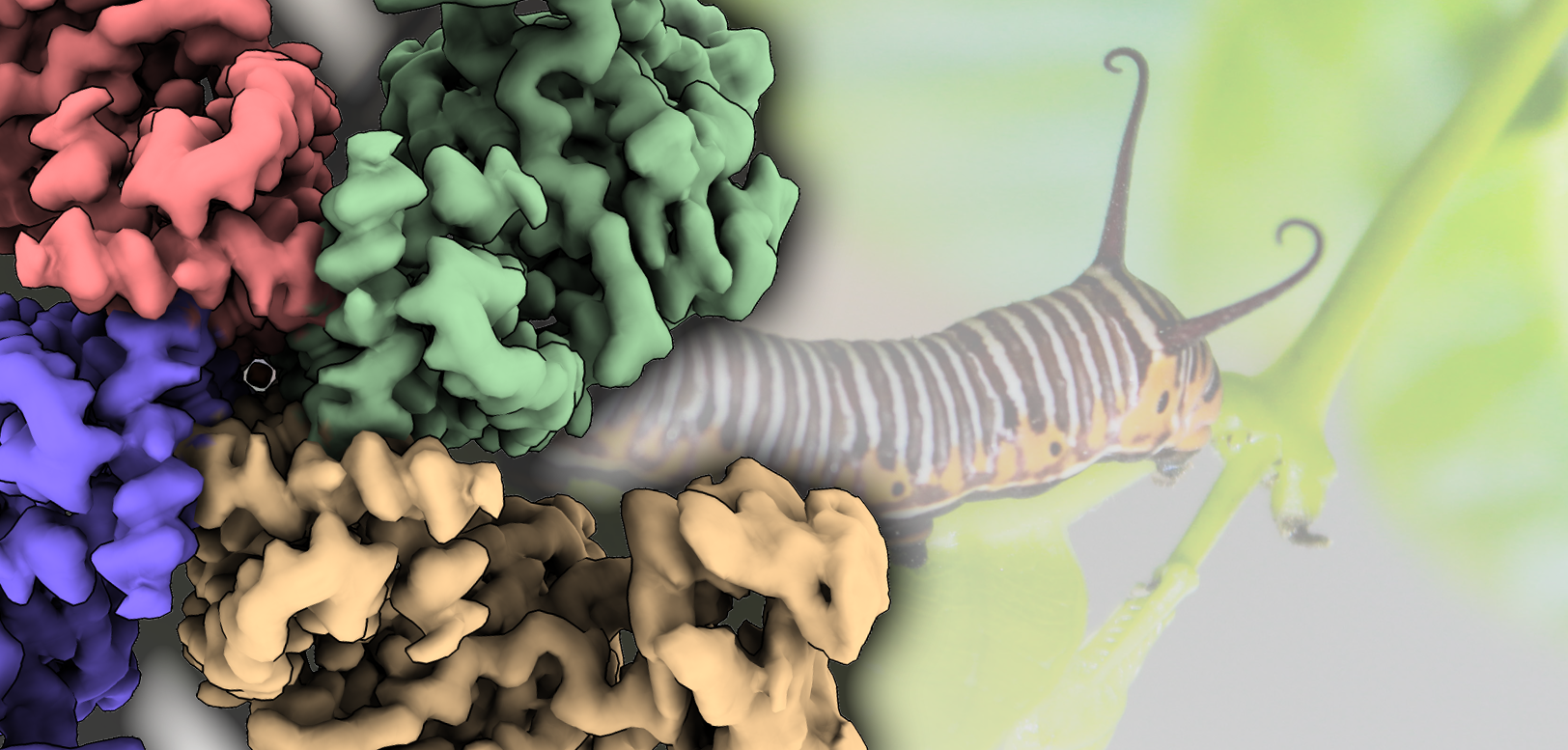
These proteins are produced as inactive protoxins that need to be activated by midgut proteases to trigger cell death. However, the understanding of their three-dimensional organization and activation mechanism at the molecular level was very limited. Nuñez-Ramírez et al. have determined the structures of the protoxin and the protease-activated state of Vip3Aa at 2.9 Å using cryo-electron microscopy. The reconstructions show that the protoxin assembles into a pyramid-shaped tetramer with the C-terminal domains exposed to the solvent and the N-terminal region folded into a spring-loaded apex. The C-terminal domain presents a cut site that is recognized by proteases of the digestive system of the insect, whose action will produce a cut that will facilitate the conversion of protoxin to toxin. After protease activation, a dramatic remodeling of the N-terminal region of the protein takes place that results in the formation of a parallel four-helix, needle-like coil. Interestingly, this nearly 160 Å needle is long enough to reach and perforate the membrane of the insect cell and produce its toxic effect.
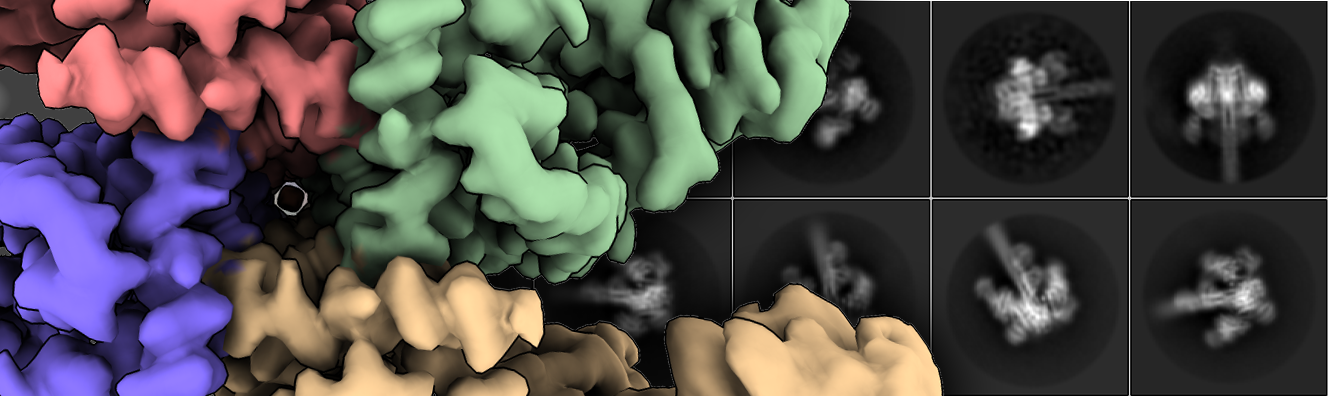
Collectively, this work reveals the structural changes that lead from the conformation of protoxin to the active form of proteins of the Vip3 family, mechanisms unknown until now despite the fact that these proteins have been studied for decades. The studies carried out help to understand how the Vip3 proteins destroy the epithelial cells of the midgut of the insect larvae and ultimately lead to their death.
Additionally, these results are a turning point in the study of the insecticidal action of Vip3A and open the door to the development of new biotechnological applications that allow the production of more stable insecticidal proteins with greater activity for the control of pests in agriculture.
Reference: Molecular architecture and activation of the insecticidal protein Vip3Aa from Bacillus thuringiensis. Rafael Núñez-Ramírez, Juanjo Huesa, Yolanda Bel, Juan Ferré, Patricia Casino, Ernesto Arias-Palomo (2020) Nature Comm. 11, 3974 (2020). https://doi.org/10.1038/s41467-020-17758-5

