A work published in the journal Protein Science by the group of Dr. Susana Camarero at Centro de Investigaciones Biológicas Margarita Salas (CIB-CSIC), reports for the first time the crystal structure of a small subunit that forms a heterodimer with the laccase-like enzyme (NLAC) of the fungus Pleurotus eryngii. The work, in collaboration with Dr. Javier Medrano also at CIB-CSIC, describes the structural features of the NLAC involved in the interaction which would relate to the different behavior of the NLAC in monomeric or heterodimeric form, as well as compared to a canonical laccase.
Fungal laccases are monomeric multicopper oxidases of great biotechnological interest due to their ability to oxidize lignin and a range of aromatic compounds. NLACs are laccase-like enzymes poorly characterized which are reported to form heterodimers with small proteins (subunits) of unknown function.
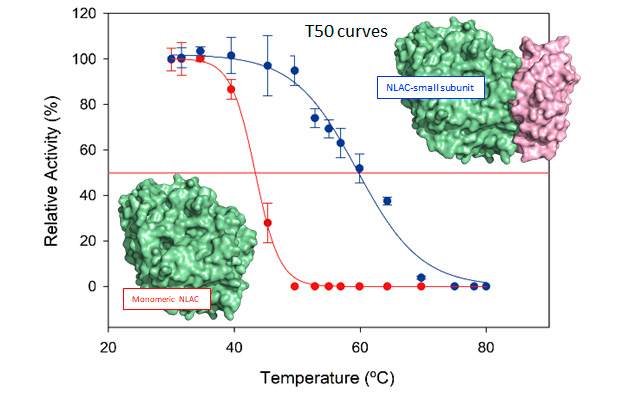
Aza et al. have heterologously produced and characterized the NLAC of the fungus Pleurotus eryngii. The enzyme forms a heterodimer with a small protein found in the fungal genome that was either co-cloned with the enzyme in Aspergillus oryzae or added to the enzyme exogenously. The heterodimer shows a remarkably enhanced stability to temperature, acid pH, and presence of co-solvents, and superior activity compared with the enzyme alone.
In this work, the authors report the first crystal structure of a small subunit of a NLAC providing evidence on the interactions with the catalytic subunit in the heterodimer. The crystallographic structure of the small protein expressed in Escherichia coli was solved at 1.6 Å resolution and allowed to describe distinctive structural features of the NLAC that are likely involved in the interaction with the helix bundle structure of the small subunit and/or in the substrate binding, which would modulate the enzymatic activity and explain the differences in activity and stability of the monomeric or complexed enzyme, and in activity of the NLAC compared to a canonical laccase.
The results obtained shed light on the structure-function of these poorly characterized non-canonical laccases and the role of the small subunits in the heterodimers formed.
Reference: Role and structure of the small subunit forming heterodimers with laccase-like enzymes. Pablo Aza, Dolores Linde, Gonzalo Molpeceres, Jesper Vind, F. Javier Medrano and Susana Camarero (2023) Protein Science. https://doi.org/10.1002/pro.4734

