The widely accepted view in the field of intracellular transport between membranous compartments is that incoming vesicles are recruited at a certain distance from the target membrane by molecular tethers, after which a set of proteins denoted SNAREs zipper up into a highly stable tetra-helical bundle that powers the fusion of donor and acceptor membranes. The combinatorial specificity of SNAREs is further improved by proteins that template the formation of the bundle. In multi-subunit tethering complexes, tethering and template activities reside in different polypeptide subunits.
In a paper recently published in the journal eLife, led by Miguel Peñalva at the Laboratory of Aspergillus Cell Biology that Dr. E.A. Espeso and himself co-direct in the Margarita Salas Center for Biological Research (CSIC), Dr. Ignacio Bravo-Plaza et al. report evidence that a protein denoted Uso1p (p115 in mammals) combines the tethering and SNARE-regulating activities in the same polypeptide chain.
The secretory pathway is a membrane-bound set of compartments that delivers proteins synthesized in the ER to the plasma membrane and the endolysosomal system after classification in the Golgi (the post office of the cell). Uso1p acts in one of the very early steps – transport between the ER and the Golgi. Yusou indeed means transport in Japanese, as Japanese were the researchers who discovered in the budding yeast Uso1p, a protein that turned out to be essential for transport across the secretory pathway.
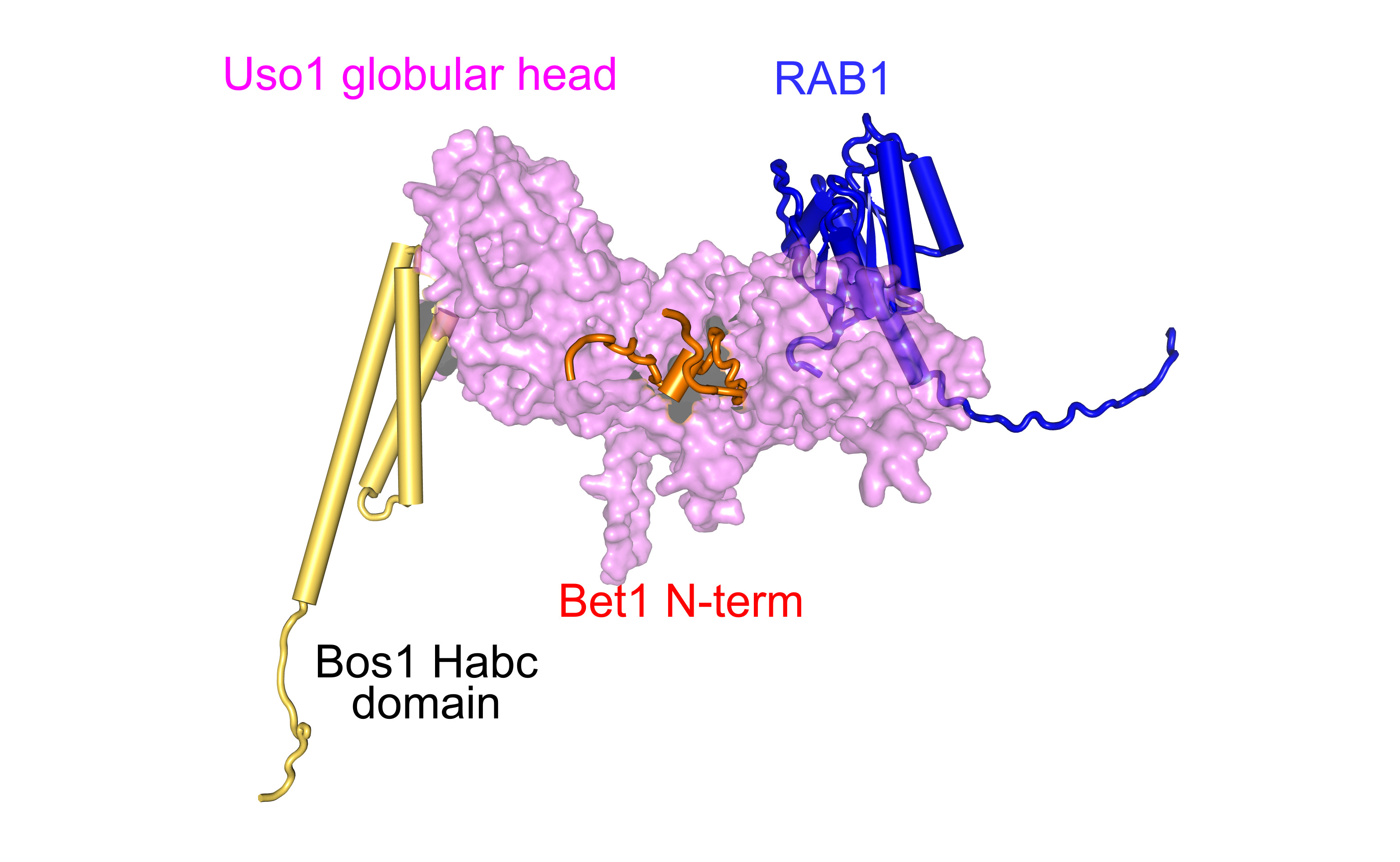
Uso1/p115 is a large protein that is recruited to membranes by RAB1 and contains an N-terminal globular head domain and a C-terminal coiled-coil domain. The presence of the latter led to the paradigm that Uso1p would act as a tether recruiting ER-derived COPII vesicles to the early Golgi. However, this view did not satisfactorily explain why other proteins mediating the tethering of vesicles to the Golgi and also containing long coiled-coils are not essential, implying that Uso1p plays another role beyond the simple tethering of vesicles.
Ignacio-Bravo Plaza, a recent doctorate from the Aspergillus Cell Biology Laboratory, used mutations generated by classical genetics to pull the string that led him and his associates to the characterization of previously unnoticed contacts between the globular head domain of Uso1 and the SNARES zippering up vesicle fusion. He concluded that Uso1p has two activities residing on the same polypeptide chain: one, located at the globular domain, which is essential and involves SNARE regulation, and a second, the non-essential activity of tethering residing at the coiled-coil.
Future experiments addressing the molecular mechanism of SNARE regulation at the atomic level should help to understand the high specificity with which ER-derived vesicles are targeted to the cis-Golgi, as yet insufficiently understood nearly 7 decades after Nobel Price George Palade described the rough ER.
Reference: The Uso1 globular head interacts with SNAREs to maintain viability even in the absence of the coiled-coil domain. Ignacio Bravo-Plaza, Victor G Tagua, Herbert N Arst, Ana Alonso, Mario Pinar, Begoña Monterroso, Antonio Galindo, Miguel A Peñalva (2023) eLife. May 30, 2023. https://doi.org/10.7554/eLife.85079

