Dr. Eduardo Rial, researcher at the Margarita Salas Center for Biological Research (CIB-CSIC), is leading a project that will test drugs used against cancer to curb infection with the SARS-CoV-2 coronavirus, which is causing the current pandemic. The project, in collaboration with María Colmenares, Luis Rivas and José María Sánchez-Puelles, also researchers from the CIB-CSIC, will study the mechanisms by which the host's cells have altered their metabolic activity as a result of the viral infection and will evaluate the efficacy of specific drugs against the virus.
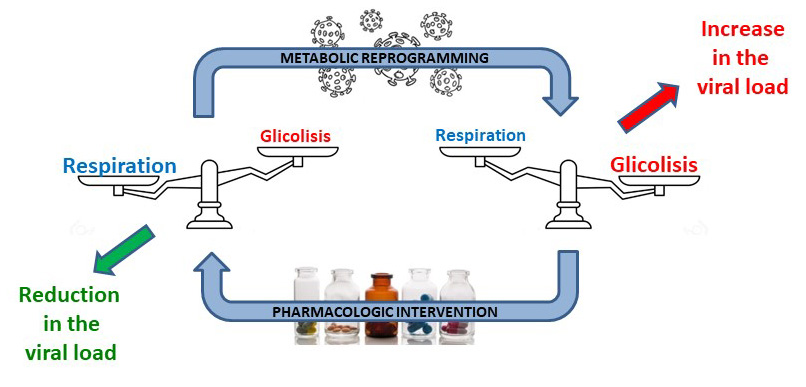
When a virus infects an organism, it hijacks the cellular metabolism of the infected cells to provide itself with the energy and precursors for the synthesis of those macromolecules necessary for the generation of new viral particles. In this way, a metabolic change is induced in the infected cell that is reminiscent of that produced in tumor cells, known as the Warburg effect.
Metabolic reprogramming is coordinated by a series of signaling routes that activate some pathways and inhibit others so that the manufacture of cellular components is prioritized. Depending on the tumor, the routes that are altered during this process are different. And in the case of SARS-CoV-2 it is still unknown which ones are affected.
The project, financed by the CSIC through the Global Health Platform, will test drugs already used clinically as anti-tumors against different pathways altered during tumor proliferation, to study their potential against the coronavirus causing COVID-19.
In this sense, one of the most interesting routes is the PI3K / AKT route, for which there are numerous drugs. Specifically, the team looks for those that produce a decrease in the production of lactic acid in the infected cell, since, as Otto Warburg observed in 1924, this is a waste metabolic product that reflects an active production of cellular components.
The researchers will use bronchial epithelial cells, infected with the coronavirus strain that produces the common cold. Next, the metabolic change produced will be observed and, in this context, the selected antitumor drugs will be tested. Drugs that provide positive results will be used by other groups in the Global Health Platform to initiate trials with SARS-CoV-2.
Repositioning of existing drugs for use against other pathologies greatly simplifies the process leading to their clinical application. Although the side effects are difficult to foresee, in the event that any of these antitumor drugs were validated as antiviral, they would not be used in long cycles, such as chemotherapy, but as acute treatment of the infection in the first days after its detection, with the goal of stopping the replication of the virus.
More information:
CSIC Press Release (in Spanish): link

