The One Earth Series
Alicia Bravo and Manuel Espinosa. Centro de Investigaciones Biológicas Margarita Salas, Consejo Superior de Investigaciones Científicas (CSIC). Ramiro de Maeztu, 9. 28040 Madrid, Spain.
This series comprises four Chapters devoted to describing the One Earth concept. We shall discuss the relationships between two separate, albeit intimate, beings sharing the same niches in many cases: Humans and Bacteria. Why are we afraid of Bacteria? Will Bacteria kill us all? How can we defend ourselves from their deadly attacks? Must we share the same niches? These and many other questions will be raised and discussed here. But we can anticipate the answer: We must share, we must co-exist. Else, Humans will be doomed.
CHAPTER II. THE BATTLE FOR BALANCE: HUMANS MEET ‘SUPERBUGS’
Introduction
In the previous Chapter, we raised the question: Must we fear bacteria? Well, we better look at the benefits and risks of our microbial world before trying to answer this question. In “normal” conditions, we keep an equilibrium between the bacteria surrounding us (inside and outside) and ourselves. And we call “health” this equilibrium. Sometimes, the balance between Humans and Bacteria is disrupted (we are not properly fed, we are stressed, or our immune system does not work properly), and it is then that we get an infection.
In other cases, we get infected by contact with other beings, like our children (especially the little ones that go to kindergartens), friends, pets, or by sharing a bus with an already infected person. This is why it is important to keep a distance when anyone is suspected of being infected. We then, in turn, become contagious and the doctor may prescribe an antibiotic. However, when we take antibiotics to fight off infections provoked by pathogenic bacteria (bacteria causing the sickness), these medications will, unintentionally, kill our commensal (beneficial) bacteria, especially the intestinal ones. This, in turn, can lead to digestive problems, lack of essential nutrients, and other health issues.
Are All Bacteria Equal?
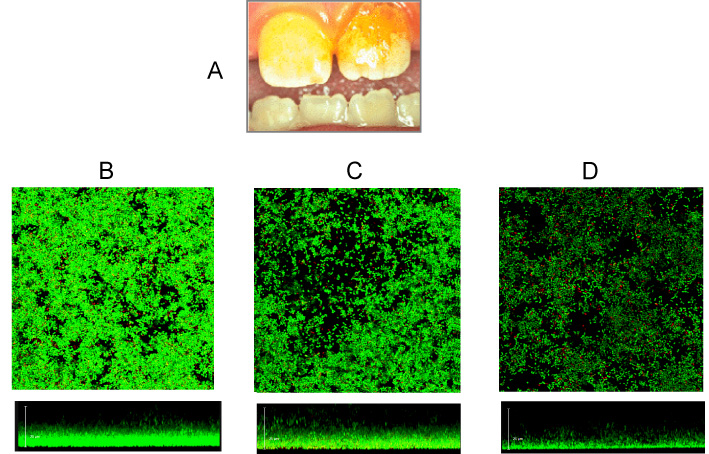
Of course, not. There are many bacterial species. Further, bacteria are ubiquitous, colonizing many different habitats (niches) on Earth, from the sea bottom to the air, arctic snow to rocks, and from deserts to rainforests. Obviously, it is not the same species that occupy the various niches: soil bacteria are different from gut bacteria, and arctic snow species do not live in rainforests, etc. In many cases, bacteria do not live like individual cells, but they live close to each other in communities called biofilms, which can be composed of one or more species (Figure 1). Frequently, some of the bacterial cells inhabiting a biofilm can release themselves and travel in fluids (urine, saliva, rivers, ponds) to “explore” other niches in the neighborhoods that can prove to be more convenient to colonize, starting then a new biofilm. However, the explorer bacteria often fail in their attempts and are eliminated. Many bacterial species are innocuous and have little relationship with other beings, except with themselves, their predators (viruses that kill bacteria, known as bacteriophages), and other microscopic competitors in their struggle for food resources.
Are All Bacteria Harmful?
We seem to be under the impression that all bacteria are harmful, which is not true: soil bacteria can be beneficial when they associate with the roots of some plants (like the leguminous family). Other bacterial species participate in fermentation in the production of cheese, yogurt, olives, wines, meats, and so on. And Humans have exploited these traits for millennia. Further, our gut microbiome provides us with essential vitamins and other compounds that contribute hugely to our well-being. However, some bacteria can pose a serious threat to our health. It is important to remember that not all bacteria are harmful, but some can cause infections that lead to severe illness. Therefore, it is crucial to protect ourselves from harmful bacteria by practicing good hygiene, such as washing our hands frequently and properly cooking and storing food. By doing so, we can greatly reduce the risk of falling ill and keep ourselves and those around us safe.
A Bit of History
The discovery of pathogenic bacteria and their potential to kill humans and animals took place around the second half of the XIX century. Around 1850, for instance, the practice of washing the hands with a chlorinated solution before attending childbirth, as recommended by the Hungarian physician Ignaz Semmelweis, showed a dramatic reduction in the rate of development of puerperal fever and subsequent deaths by women and children. Even at the time, Semmelweis did not know the existence of bacteria and attributed the deaths to some “particles” from corpses that were transmitted to the patients and the doctors attending childbirth. But it was later, when Louis Pasteur, Robert Koch, and other researchers discovered bacteria, and their relationships with diseases that the concepts of hygiene in the avoidance of infectious diseases started. After these findings, bacterial infections took an ugly look and triggered a long-term fight against the “deadly enemies” that have been taking place over the years until today. There was a peak of justified optimism on a “total victory” at the time of the discovery and first uses of antibiotics around the 1940s. In February 1941, the first person to receive penicillin was an Oxford policeman who was exhibiting a serious infection with abscesses throughout his body. However, soon afterward (in 1947), bacteria carrying penicillin-resistance genes were found and a race started (new antibiotic a new resistance a new antibiotic, etc.) that has been worsening with time until today.
And Humans Met The Superbugs
The years of use, abuse, and misuse of antibiotics in hospitals, animal husbandry, chicken farms, fisheries, and even agriculture (Figure 2) have led to the selection of bacterial variants that are resistant to them. Thus, bacteria that are resistant to many antibiotics have been selected over the years. They are called ‘superbugs’ because of this multiple resistance. Furthermore, we have learned that genetic transfer processes can spread antibiotic resistance from one bacteria to others of the same, or different, species.
For several years, an antibiotic termed vancomycin was used in the treatment of infections caused by bacteria resistant to multiple antibiotics. Due to this reason, vancomycin was considered as a “last-resort antibiotic”. However, the emergence of bacteria resistant to vancomycin sounded all alarm bells. Among these resistant bacteria, one called Enterococcus faecalis caused much concern. This bacterium is naturally resistant to many antibiotics and it is prone to acquire new resistance genes. Normally, it peacefully coexists with other bacteria in our gut without causing harm (commensal behavior). However, it may gain access to other parts of the body like the urinary tract, heart, or bloodstream. In such niches, it may cause infections that vary from mild to life-threatening, especially in elders, children below 5, and patients in hospitals: this is to say humans with an immune system that is compromised and does not function correctly. How to treat these infections if the bacteria causing them are resistant to multiple antibiotics?
Infections acquired during a hospital stay are called “nosocomial” infections (from the Latin “nosocomium”, hospital). There are instances of commensal bacteria that escape from their usual niches to colonize new ones. They take advantage of a weakness in our immune system for their escape and they are called “opportunistic bacteria”. Many infectious diseases are due to opportunistic bacteria. Isolation and characterization of superbugs commenced by the end of the 20th Century. There were some attempts to commercialize new antibiotics, like the so-called “Initiative 20-20” which intended to market 20 new antibiotics by the year 2020, but there was not much success. In 2022, the rate of loss of human lives caused by superbugs in Spain was more than 23,000. And the numbers seem to be increasing.
And so, we conclude with a doleful question: How have we arrived at the present situation?
Further Readings:
In Spanish:
In English:
Acknowledgements
This series attempts to communicate our research at the Centro de Investigaciones Biológicas Margarita Salas, CSIC and is part of the Grant I+D+i PID2019-104553RB-C21, funded by MICIU/AEI/10.13039/501100011033. Thanks are due to María del Carmen Fernández and Mónica Fontenla for their help during the elaboration of this series.
Other chapters:
Chapter I: link.
Chapter III: link.
Chapter IV: link.

