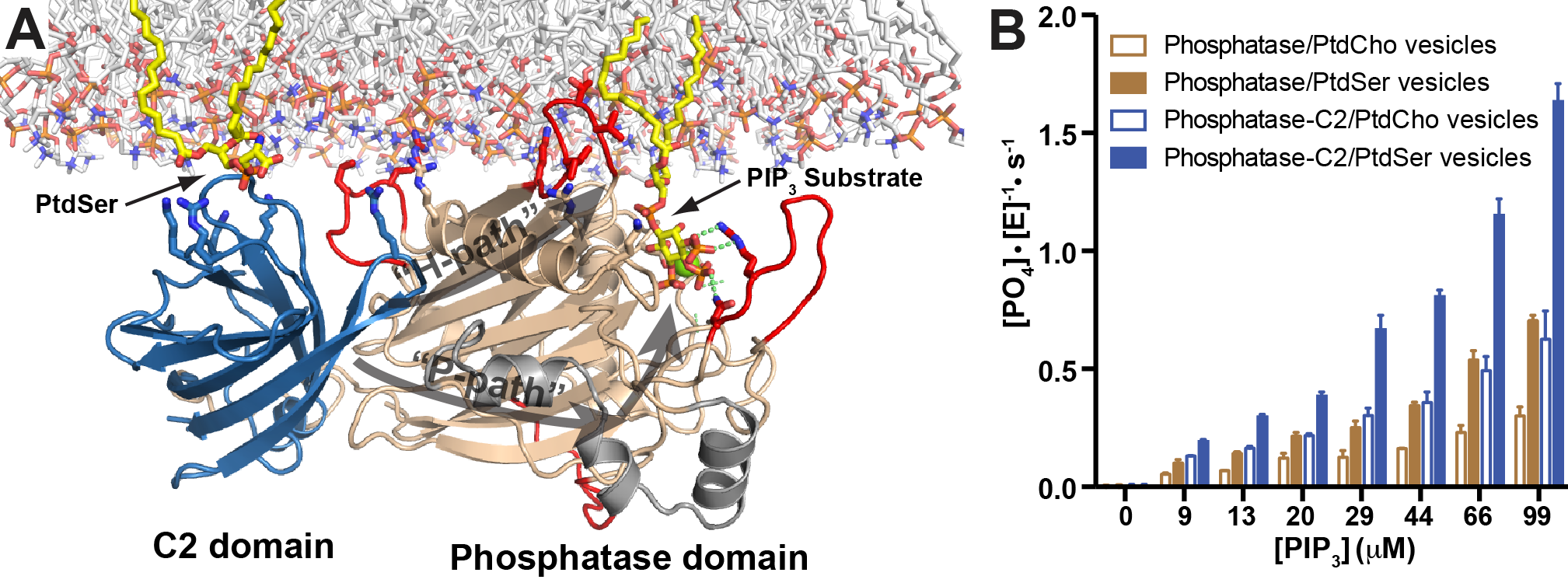
SH2 containing inositol phosphatase 2 (SHIP2) removes the 5-phosphate from the PI(3,4,5)P3 lipid and thereby, like PTEN, negatively regulates PI(3,4,5)P3 levels in the plasma membrane. Despite their importance in physiology and disease little is known about mechanisms of SHIP regulation. We solved a crystal structure containing the catalytic and C2 domains of SHIP2, which in combination with biochemistry and cell biology showed how the C2 domain induces catalytic activation of SHIP2 (Figure). We further studied the role of the PH-related (PHR) domain flanking the catalytic domain and found that the domain binds the PI(3,4,5)P3 substrate and PI(3,4)P2 product and this binding allosterically further activates SHIP2. Together, this shows how the C2 and PHR domains concertedly act to recruit SHIP2 to PI(3,4,5)P3 rich membranes to adopt a highly active state.