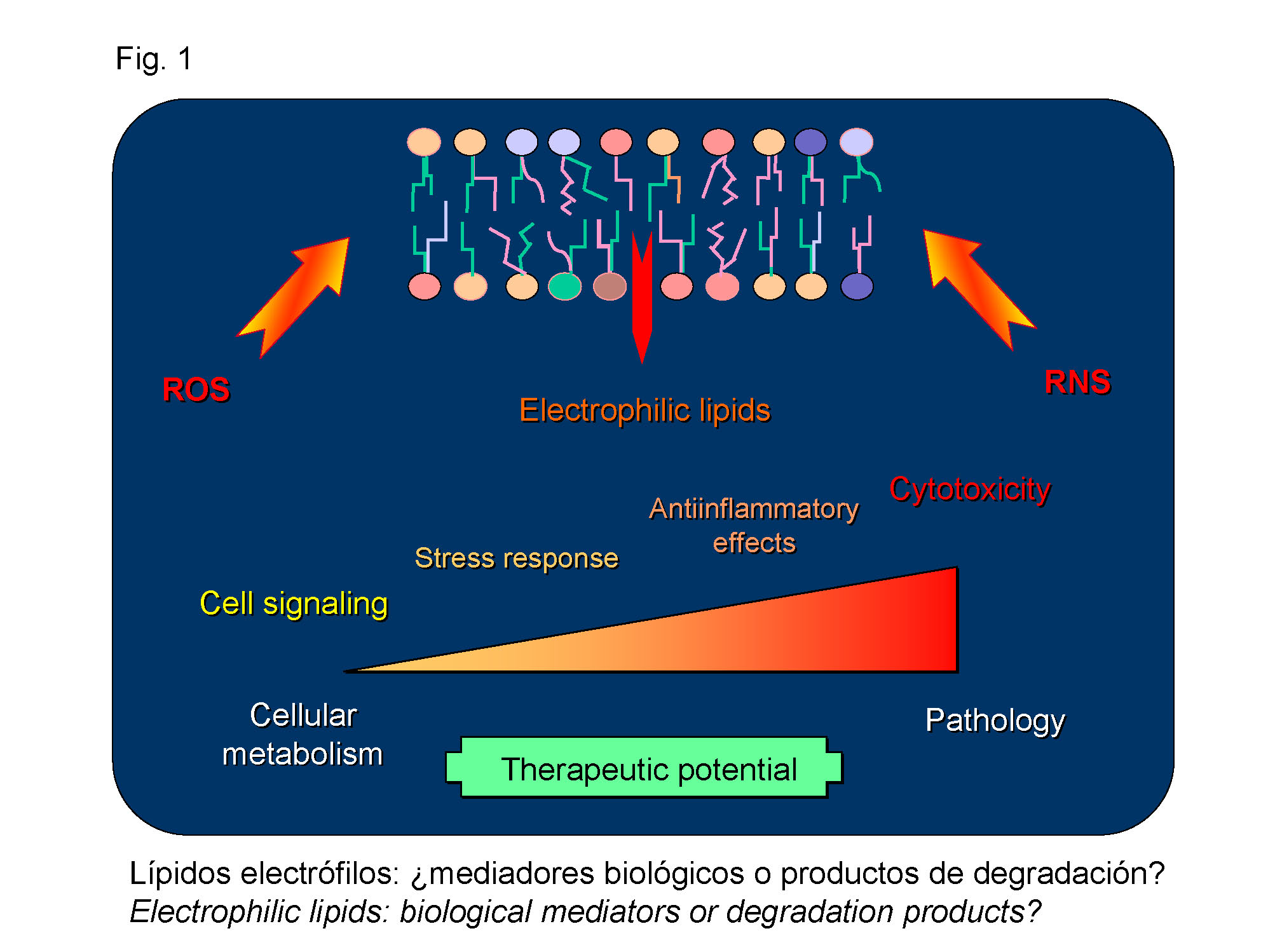
Electrophilic lipids are reactive biological mediators that are generated through the action of reactive oxygen or nitrogen species on cellular lipids, mainly polyunsaturated fatty acids. Electrophilic lipids possess diverse structures and can act by multiple mechanisms, including their interaction with cellular receptors and the direct modification of proteins (protein lipoxidation). In low physiological concentrations, lipid electrophiles contribute to cell defence mechanisms, moderate concentrations display antiinflammatory and antitumoral properties. However, in pathophysiological situations associated with oxidative stress, increased levels of electrophilic lipids can contribute to the onset or the progression of diseases such as atherosclerosis or neurodegenerative diseases (Figure 1).
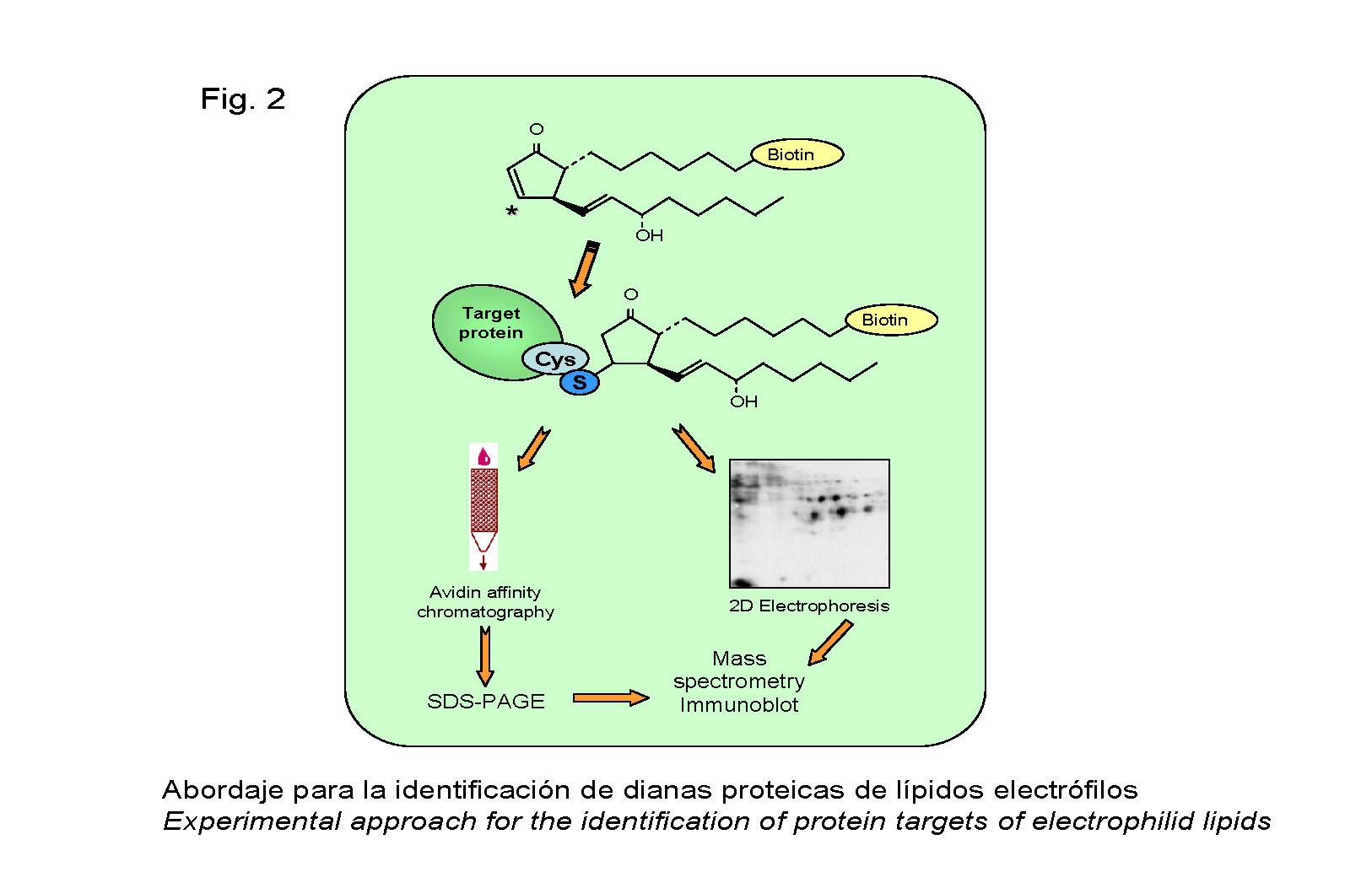
We have developed methods for the identification of the protein targets for direct modification by lipid electrophiles through proteomic approaches, and we are carrying out the functional and structural characterization of the modified proteins (Figure 2).
Among the protein targets we have identified vimentin, the protein that forms intermediate filaments. Vimentin is modified at its only cysteine residue. The function of this residue was previously unknown. Our results indicate that it is important for cytoskeletal organization during the cell response to stress (see Figure 3 and Stamatakis et al., 2006, J. Am. Soc. Nephrol. 17, 89-98).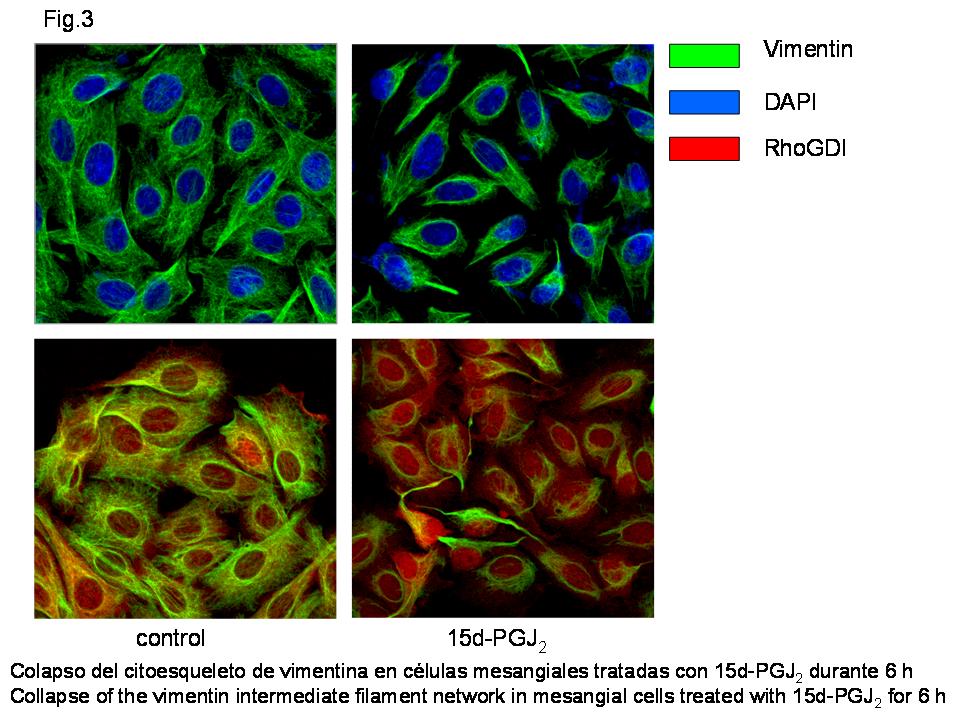
By using electrophilic lipids with diverse structures we have observed that, in spite of their broad reactivity, protein modification by these compounds is selective, since compounds with diverse structure induce different modification patterns, both in intact cells and in isolated proteins.
These observations open new avenues for the development of more specific molecules. Our studies may contribute to the identification of novel therapeutic targets and to the characterization of the functional consequences of protein modification by lipids.

