Tubulin modulating agents interfere with microtubule dynamics, thus altering the: 1) cytoskeleton, 2) the cell division and 3) the transporting functions of different cargoes over them. Although the molecular effects on the target protein are getting progressively better characterized, the way in which these effects are translated into cellular responses remains in many cases unclear.
We do analyse the effect of tubulin modulators in the cytoskeletal structure and the cell division using classical IC50s, flow cytometry, inmunofluorescence and, more recently, time-lapse studies on cells with fluorescently tagged proteins. For the study of the effect in cargo transport, we complement the previous analysis with the current implementation of time-lapse analysis of quantum-dot labelled kinesins and dyneins movement on microtubule bundles in the absence/presence of different compounds.
Although it is assumed that microtubule targeting agents exert its antitumoral effect through cell division blocking, it is not clear how an agent that blocks cell proliferation promotes potent tumour regression even in low proliferating cancers.
To enlight this question we are investigating the fate of different types of cells (tumoral, immortalized, p53 +/-) after being exposed to different tubulin modulating agents as well as the effect of these agents in activating and/or adapting to the Spindle Assembly Checkpoint (mitotic slippage). Cells with perturbed tubulin are unable to properly biorient chromosomes at metaphase and thus activate the Spindle Assembly Checkpoint, which blocks cells in c-mitosis until all sister kinetochores are bioriented toward opposite poles and ready to separate sister chromatids synchronously. Those blocked cells after prolonged arrest can follow different fates: die by apoptosis in mitosis, slip from the block and die at the beginning of the next cycle as tetraploid cells or follow a new cell cycle as cells carrying aneuploid nuclei or follow senescence.
Another problem we are working with is the understanding of the cellular reasons of peripheral neurotoxicity associated to tubulin targeted chemotherapy. To do so we are developing an in cell system to analyse the movement of kinesins and dyneins along axons of cell cultured neurons treated with different kind of compounds based on the hypothesis that microtubule stabilizing agents that do not modify the microtubule lattice will show less interference with axonal transport by dyneins/kinesins and thus less neurotoxic effects.
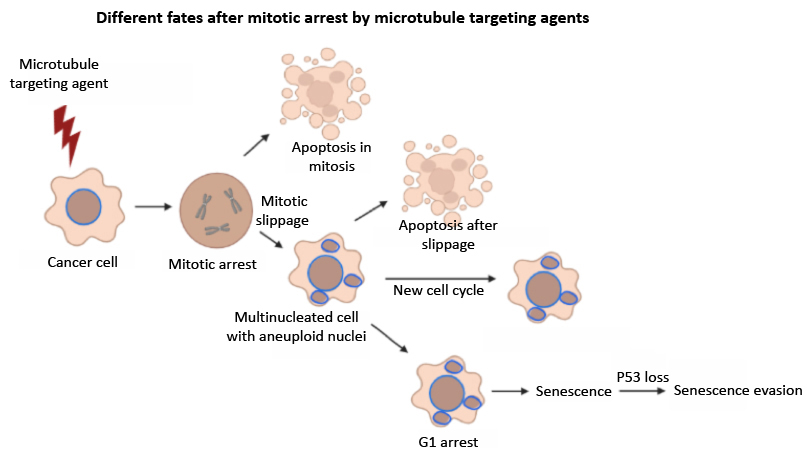
Figure:Cartoon depicting the different fates proposed in the literature for c-mitotic arrested cells after using antimicrotubule agents. Cells can undergo apoptosis directly from c-mitosis or from the beginning of a new cell cyle. Cells escaping apoptosis can enter and progress through a new cell cycle or they can remain blocked in G1, entering senescence afterwards, which could be evaded upon p53 loss.

