Ageing is a process by which basic cellular functions and tissue homeostasis start to decline and organs become progressively dysfunctional. Aged tissues are enriched in senescent cells which communicate with other neighbouring cells via the senescence-associated secretory phenotype (SASP). This SASP is enriched in soluble factors, metabolites and extracellular vesicles. In our lab, we are interested in understanding how this secretome is regulated and how it influences the surrounding cells and the microenvironment in different conditions.
On one hand, we have found that small extracellular vesicles (sEV) mediate paracrine senescence in normal proliferating cells (Figure 1). A comparison analysis show similarities between soluble factor-mediated and sEV-mediated paracrine senescence. However, sEV contained very few soluble SASP factors. An interferon protein, IFITM3, was found to be enriched in sEV from senescent cells and partially responsible for inducing paracrine senescence (Cell Reports 2019). Furthermore, we have unveiled that sEV mediate paracrine senescence via activation of the NF-kappa/IKK pathway (Trends Cell Biol 2021).
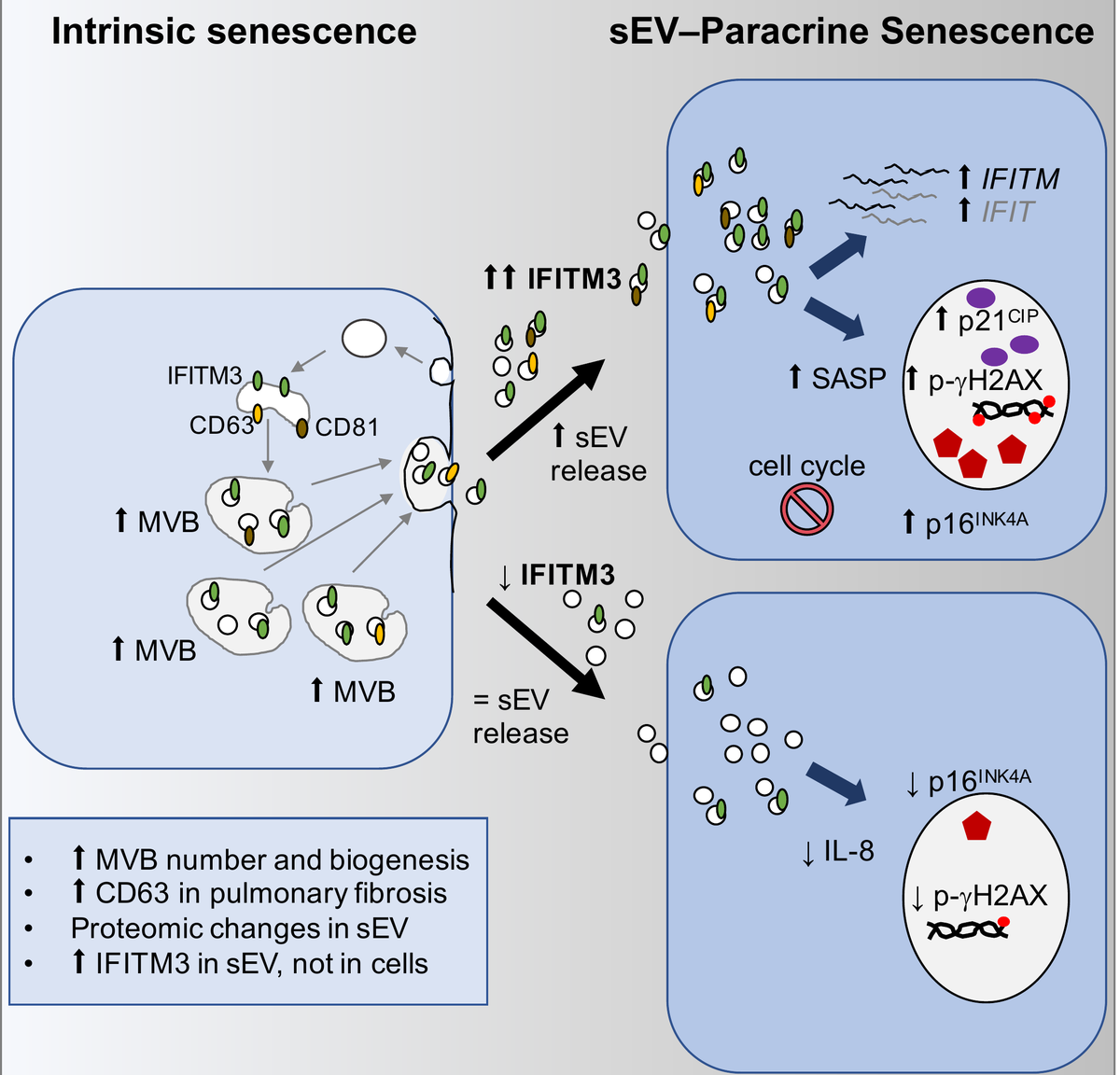
Figure 1. Small extracellular vesicles isolated from senescent cells mediate paracrine senescence
Interestingly, we have found that sEV isolated from young cells present anti-ageing and rejuvenation properties. Importantly, we found that sEV can act as independent metabolic units. Via the glutathione-S-transferase (GST) activity they can ameliorate different features of senescence and ageing both in vitro and in vivo (Figure 2) (Cell Metabolism, 2020a, Trends Cell Biol 2020b, Nat Rev Mol Cell Biol 2020 and Cell Cycle 2022).
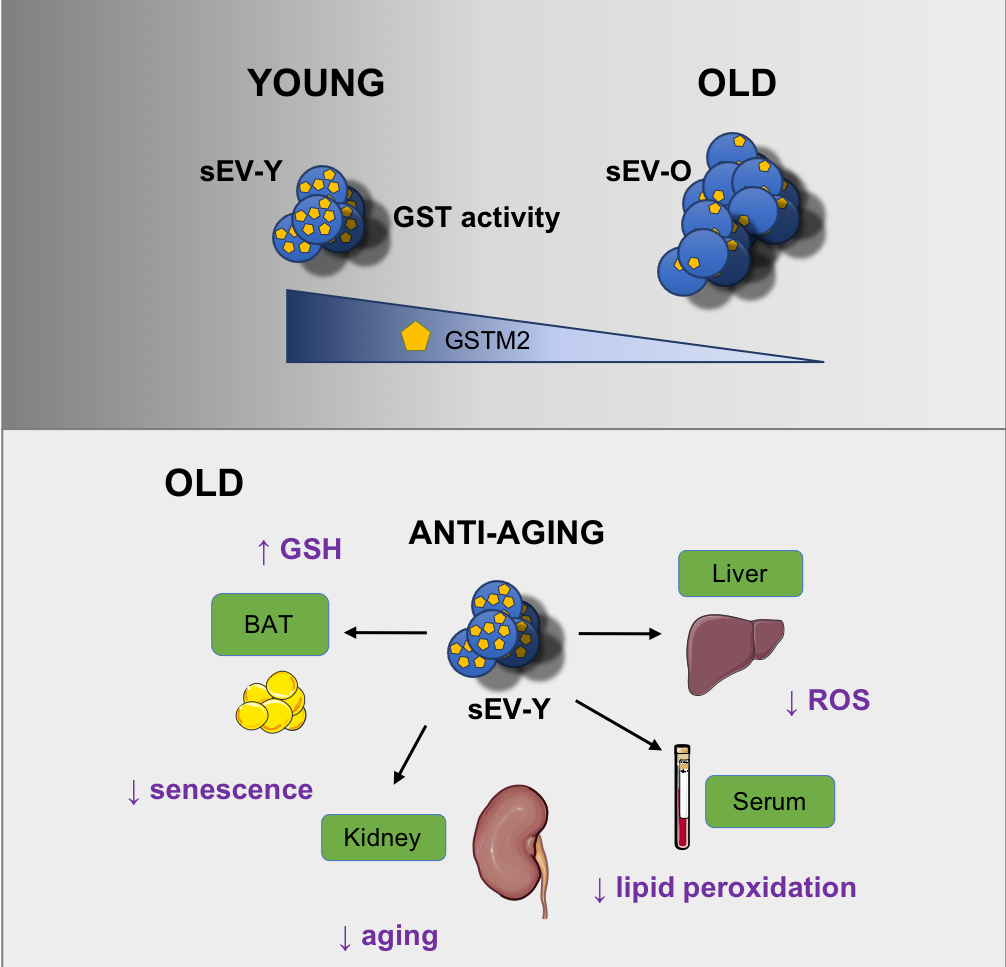
Figure 2. Small extracellular vesicles can mediate rejuvenation in old mice via the glutathione-S-transferase M2 (GSTM2) protein

