Investigation into biodegradable polyesters has a great impact on the biomedical sector. A work published in the International Journal of Biological Macromolecules and carried out by Dr. Auxiliadora Prieto’s group at the Centro de Investigaciones Biológicas Margarita Salas (CIB-CSIC) led to the development of small material binding peptides (MBPs) inspired by nature. The authors are part of the Interdisciplinary Platform SusPlast (from CSIC), whose mission consists of transforming the design, production, use, and recycling of plastics to implement plastics management based on a circular economy.
Synthetic polyesters, such as PET, have been used for a long time in the healthcare sector, particularly for the manufacture of biomedical implants. This is due to its high strength, durability, and versatility properties that can be comparable to many tissues in the body. Nevertheless, their fossil origin, chemically inert nature, and resistance to biodegradation could cause certain drawbacks, such as hindering cell adhesion or implant resorption, among others. For this reason, biopolyesters may represent a more suitable option when biodegradability is required.
Polyhydroxyalkanoates (PHAs) are polyesters produced in nature by several microorganisms including bacteria. They may represent an alternative to synthetic polymers in various medical applications such as sutures, implants, and drug delivery systems. However, despite their advantageous properties, PHAs are usually produced as polymers that lack complex functional groups. For this reason, there is currently growing interest in functionalizing them, in order to expand their range of application. PHAs functionalization consists of incorporating specific functional groups that confer certain characteristics. Surface functionalization of PHAs with peptides, proteins, or even entire cells has been suggested as a versatile and straightforward approach to improving the potential of PHAs for biomedical applications. A sophisticated strategy relies on connecting target molecules (e.g., bioactive proteins) into small anchoring peptides with inherent material-binding properties, obtaining a direct assembly of bioactive molecules onto several biomaterials.
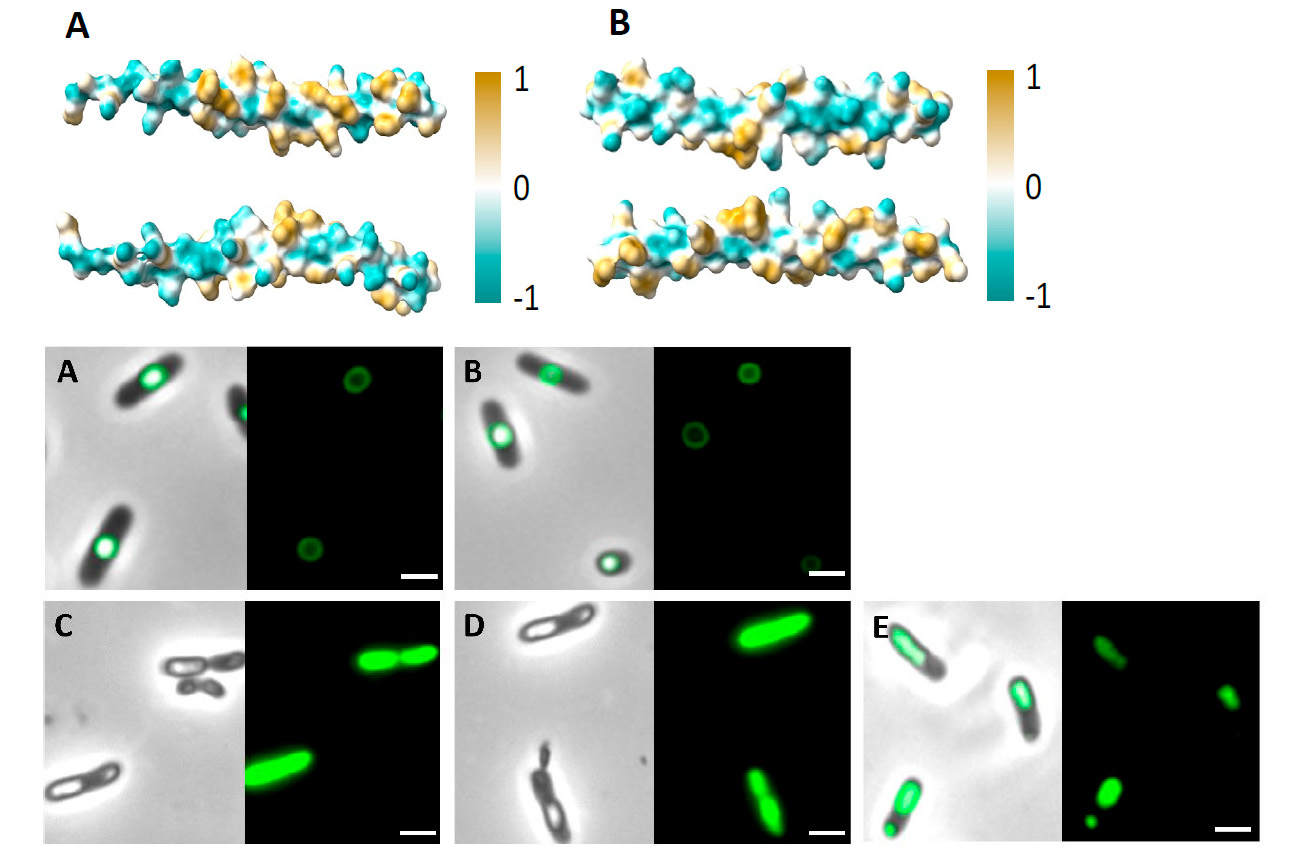
In this work, the authors designed and generated two MBP of 48 amino acids, which can assemble bioactive molecules into biomaterials such as PHAs. For this, they got inspiration from phasins, a group of bacterial proteins that have a high affinity towards PHAs. “Inspired by granule-associated proteins existing in nature, such as phasins, we generated small MBPs for polyester surface functionalization”, write the authors.
Inside the cell, phasins bind to cytoplasmic granules of PHAs. This property is imparted by the accumulation of hydrophilic and hydrophobic residues on two opposite sides of the protein, which gives them an amphiphilic character. “The peptides generated in this work, called MinP and MinI and derived from the phasins PhaF and PhaI of the bacterium Pseudomonas putida, were designed following a rational approach based on amphiphilicity,” explains Ana M. Hernández-Arriaga, researcher at the Dr. Prieto’s group and co-author of the work. “Both peptides presented a high binding affinity towards the two types of PHA evaluated (PHB and PHOH) and have the ability to immobilize proteins on the surface of the polyester, which makes them ideal candidates for the functionalization of PHA,” she adds.
The binding affinity of both peptides towards PHAs was evaluated in vivo -inside the bacterial cytoplasm- through fluorescence studies (using the green fluorescent protein, GFP), and in vitro, through a biophysical approach, using the Langmuir-Blodgett technique coupled to ellipsometry.
“Furthermore, we demonstrated the capacity of both MBPs to bind to other synthetic polyesters, such as PET, supporting future applications for small peptides in fields beyond biomedicine”, write the authors.
This work has been carried out in collaboration with Natalia Tarazona Lizcano’s lab at the Institute of Functional Materials for Sustainability of the Helmholtz Hereon Centre and it is part of Francisco Blanco Parte’s doctoral thesis.
Reference: Nature-inspired material binding peptides with versatile polyester affinities and binding strengths. F. G. Blanco, R. Machatschek, M. Keller, A. M. Hernández-Arriaga, M. S. Godoy, N. A. Tarazona, M. A. Prieto. DOI: 10.1016/j.ijbiomac.2023.126760

