A study published in the Journal of Thrombosis and Haemostasis by Dr. Carmelo Bernabeu's group in collaboration with Dr. M. Cristina Vega, both researchers at the Margarita Salas Center for Biological Research (CIB-CSIC), shows that a circulating form of endoglin, known as soluble endoglin (sEng), acts as a new regulator of primary hemostasis and thromboinflammatory processes. This finding is the result of an international collaboration that has also involved the teams of Dr. Elisa Rossi from Paris Cité University (France) and Dr. Miguel Pericacho from the University of Salamanca.
sEng is a cleavage product of membrane-bound endoglin expressed in the endothelium. Previous studies have described elevated plasma sEng levels in several diseases related to the circulatory and cardiovascular systems, including atherosclerosis, preeclampsia, myocardial infarction, diabetes, and hypertension. However, their role in the process of hemostasis was unknown. Because platelets play an essential role in integrin-mediated hemostasis and because sEng contains an Arginine-Glycine-Aspartic acid (RGD) sequence involved in integrin binding, the authors hypothesized that sEng might bind to the platelet integrin αIIbβ3. This integrin is critical for platelet binding to fibrinogen and for thrombus stability.
Rossi et al. performed in vitro human platelet aggregation, thrombus retraction, and secretory competition assays in the presence of sEng. Under flow conditions, supplementation of human blood with sEng led to smaller thrombus size. In addition, sEng inhibited platelet aggregation and thrombus retraction by interfering with fibrinogen binding, but without affecting platelet activation.
Computational and surface plasmon resonance (SPR) binding analyses allowed the evaluation of protein-protein interactions. SPR binding studies demonstrated specific interaction between αIIbβ3 and sEng and molecular modeling showed a good fit between αIIbβ3 and sEng structures involving the RGD motif of sEng, suggesting the formation of a highly stable αIIbβ3/sEng.
Additionally, a transgenic mouse overexpressing human sEng (hsEng+) was used to measure bleeding/rebleeding, prothrombin time (PT), and embolus formation after FeCl3-induced carotid artery injury. Thus, hsEng+ mice showed increased bleeding time and number of rebleeds compared to control mice. However, no differences in PT were observed between genotypes. After FeCl3 injury, the number of emboli released in hsEng+ mice was higher and occlusion was slower compared with controls.
These results demonstrate that sEng interferes with thrombus formation and stabilization, probably through its binding to platelet αIIbβ3, indicating its involvement in the control of primary hemostasis, and opening new therapeutic avenues in thromboinflammatory processes.
Reference: Soluble endoglin reduces thrombus formation and platelet aggregation via interaction with αIIbβ3 integrin. Rossi E, Pericacho M, Kauskot A, Gamella-Pozuelo L, Reboul E, Leuci A, Egido-Turrion C, El Hamaoui D, Marchelli A, Fernández FJ, Margaill I, Vega MC, Gaussem P, Pasquali S, Smadja DM, Bachelot-Loza C, Bernabeu C. J Thromb Haemost. 2023 Mar 27: S1538-7836(23)00254-4. doi: 10.1016/j.jtha.2023.03.023
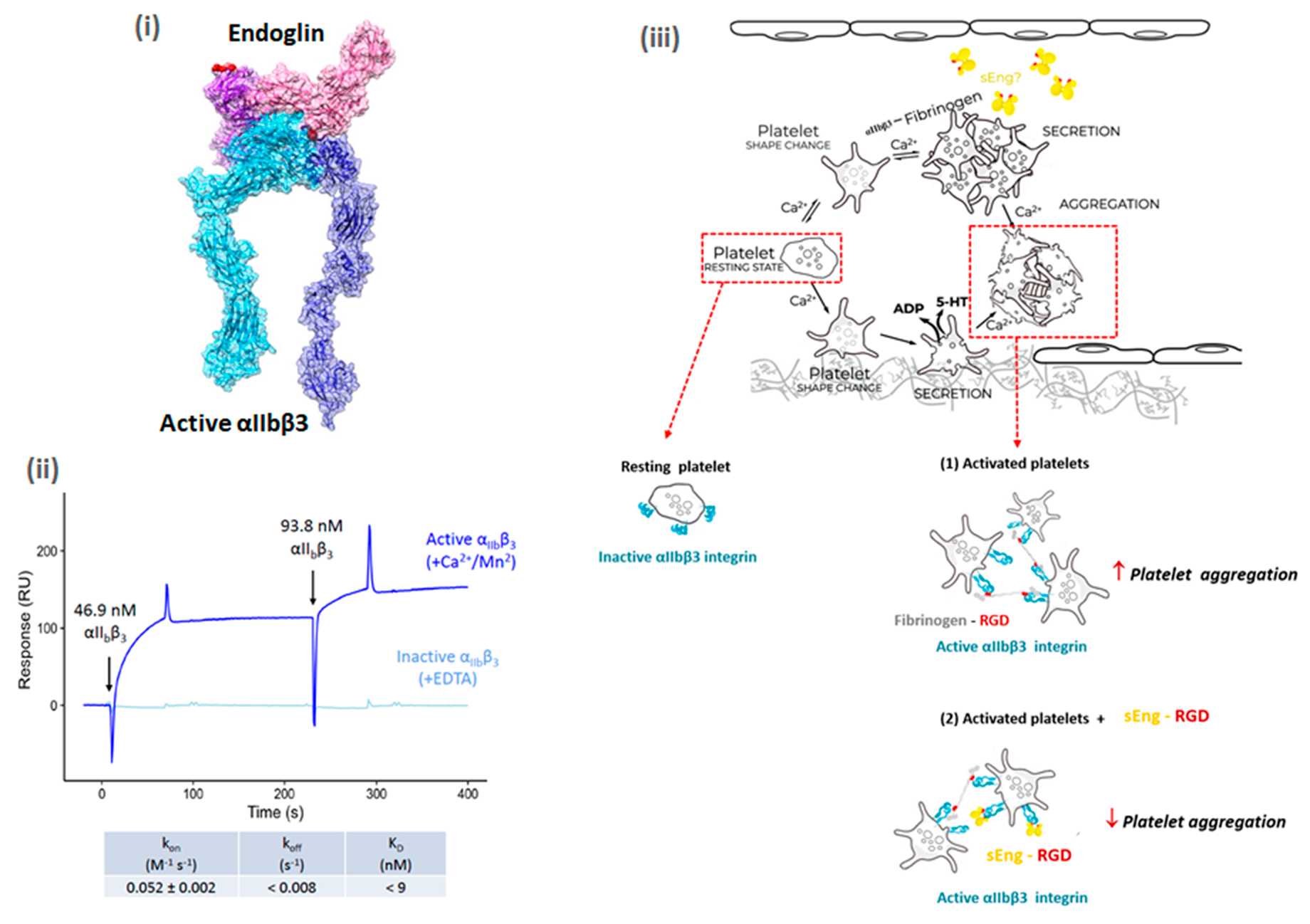
[FIGURE CAPTION]
(i) Structural modeling (Docking) of the complex between active integrin αIIbβ3 (dark-light blue) and sEng (dark-light pink). The sEng RGD motif is marked in red.
(ii) Binding analysis of sEng and active or inactive integrin αIIbβ3 by surface plasmon resonance. The sensorgram corresponds to the first two consecutive injections of the ligand (integrin αIIbβ3) over the sensor chip immobilized with sEng.
(iii) Hypothetical mechanism of sEng-induced inhibition of platelet aggregation. Circulating platelets are recruited to the injury site via adhesive interactions, including the binding between membrane-bound endothelial endoglin and platelet αIIbβ3. Upon activation, platelets undergo secretion and aggregation to one another involving integrin αIIbβ3-mediated interactions of platelets with fibrinogen. Membrane-bound endoglin can be proteolytically processed to yield a soluble form of endoglin (sEng) that contains an RGD motif. Resting platelets expose inactive integrin αIIbβ3 on their surface, but after platelet stimulation, integrin αIIbβ3 is activated enabling the formation of fibrinogen-αIIbβ3 interactions mediated by the RGD motif leading to platelet aggregation (1). sEng binds to αIIbβ3 via its RGD motif, inducing a destabilization of the thrombus by “interfering” with fibrinogen-αIIbβ3 interactions and thus inhibiting platelet aggregation (2).

