A work recently published in the journal eLife by the group led by Dr. Fernando Díaz at Centro de Investigaciones Biológicas Margarita Salas (CSIC) has shown for the first time the crystallographic structure of taxol derivatives in complex with tubulin. The study, the result of an international collaboration, describes their interaction in atomic detail to assess the structural determinants for binding.
The taxane paclitaxel (Taxol®) is one of the most important, first-line anti-tubulin agents used in chemotherapy to date. Listed as one of the drugs in the WHO “Model List of Essential Medicines” and despite its high value for the treatment of solid tumors, undesired neurotoxic and cell resistance side effects often hamper Taxol’s full potential.
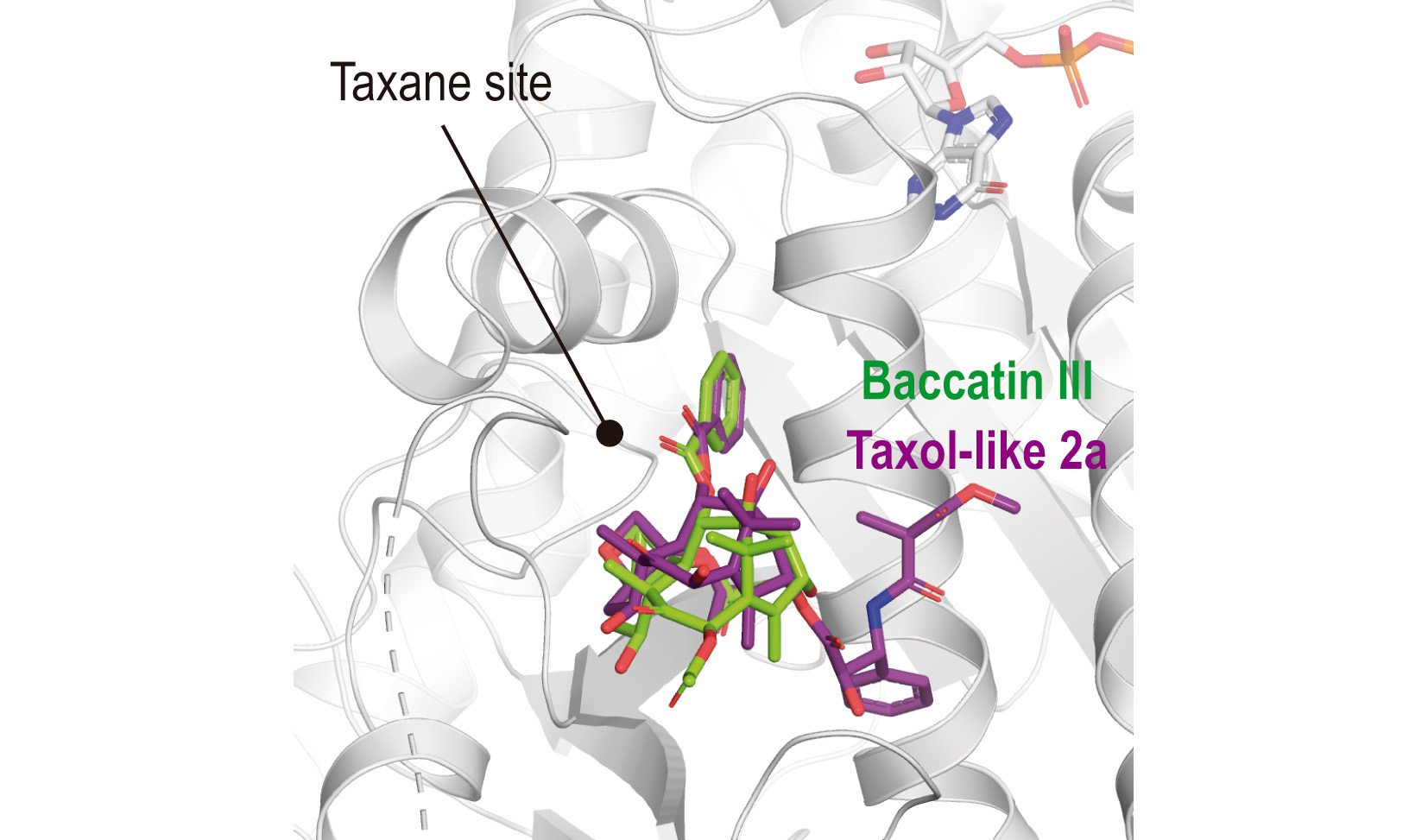
It is well known that Taxol acts by stabilizing microtubules. In this context, understanding the underlying mechanism of microtubule formation and stabilization by taxanes at high resolution would lay down a framework for the development of future and safer drugs. However, up to now no detailed information at the atomic level was available, neither on the Taxol-interacting network within the taxane site of tubulin nor on the impact of taxane binding on the structure of microtubules. Problems to crystalize the tubulin-Taxol complex in the past have been linked to the hypothesis that the mechanism of action of taxanes purely involves the binding to straight tubulin, thus preventing Taxol to bind the curved tubulin conformation present in the available tubulin-crystal systems.
Prota et al. have solved these problems by generating Taxol derivatives, by chemical synthesis, that are able to overcome this limitation, and thus have obtained the first high-resolution crystal structures (down to 1.9 Å resolution) of several taxanes bound to tubulin. The combination of experimental and computational approaches has allowed to understand the mechanism of microtubule stabilization by taxanes at the atomic level and the chemical determinants responsible for the binding and the structural distortion introduced in microtubules when stabilized with paclitaxel.
Moreover, one of the main undesired secondary effects of taxanes is peripheral neurotoxicity. The most supported hypothesis is that the structure distortion induced by paclitaxel binding affects the chemical transport along the neurites, resulting in toxicity. The researchers have found that this microtubule distortion is not related to the stabilization effect, being therefore possible to design taxanes able to stabilize microtubules without distorting their structure.
Altogether, the results point out that such taxane derivatives would possibly have the ability to treat cancer with lower toxicity and to be employed as Tau mimetics in neurodegenerative tauopathies such as Alzheimer's disease.
Reference: Structural insight into the stabilization of microtubules by taxanes. Andrea E Prota, Daniel Lucena-Agell, Yuntao Ma, Juan Estevez-Gallego, Shuo Li, Katja Bargsten, Fernando Josa-Prado, Karl-Heinz Altmann, Natacha Gaillard, Shinji Kamimura, Tobias Mühlethaler, Federico Gago, Maria A Oliva, Michel O Steinmetz, Wei-Shuo Fang, J Fernando Díaz (2023) eLife. 12:e84791. https://doi.org/10.7554/eLife.84791

