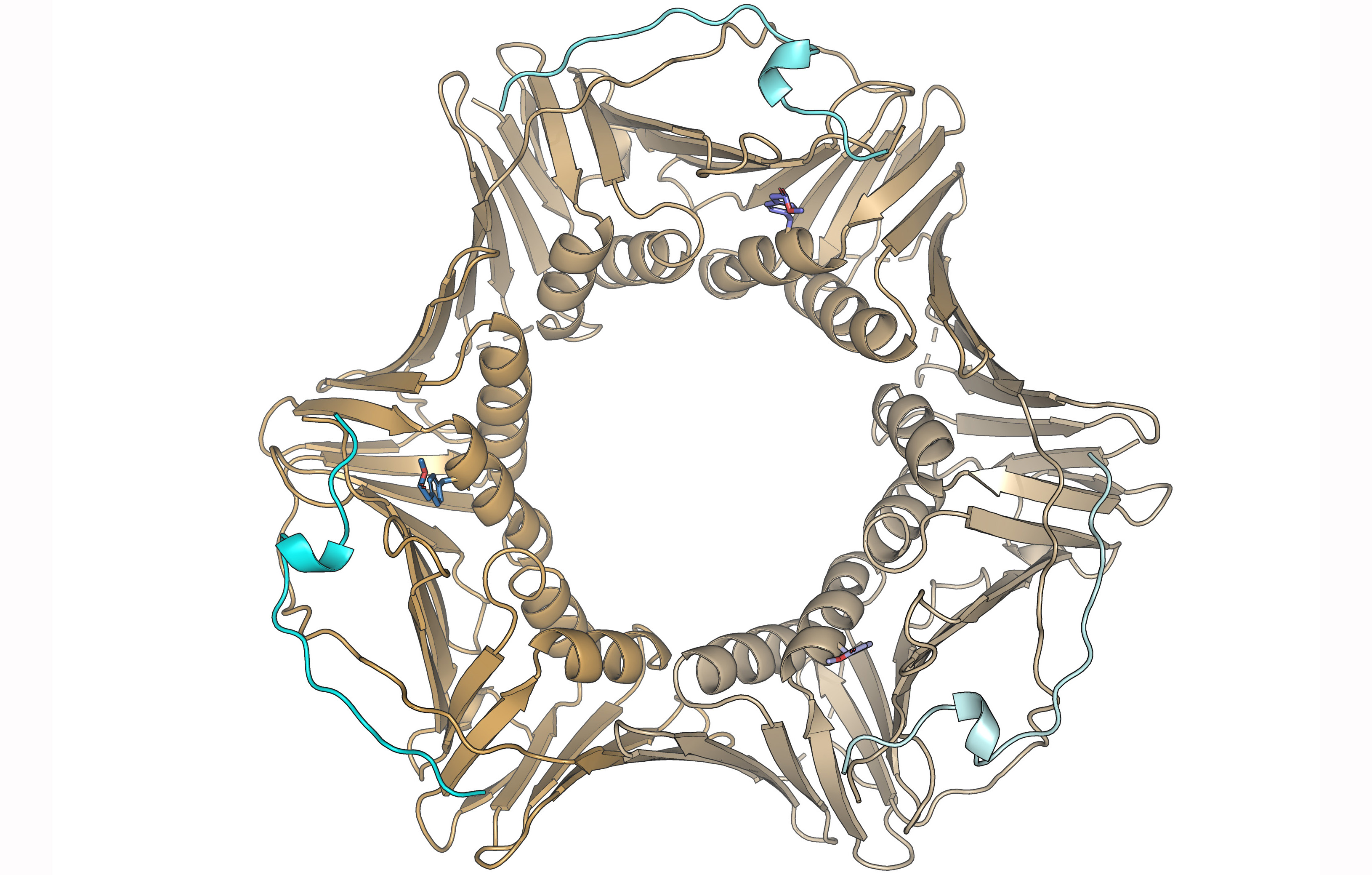
A paper published in the International Journal of Biological Macromolecules by the Biomolecular NMR group of the Margarita Salas Center for Biological Research (CSIC), led by Dr. Francisco J. Blanco, describes the production of the PCNA protein by incorporating a non-natural amino acid that mimics phosphorylation at tyrosine residues and its effect on the molecular recognition of regulatory proteins of DNA replication and repair.
Ruíz-Albor et al. incorporated the amino acid para-carboxymethyl-L-phenylalanine into the native Tyr211 position of human PCNA produced in bacterial cultures by co-expression with a tRNA reading the amber stop codon and the enzyme that binds to this amino acid, added to the culture medium. The incorporation, confirmed by mass spectrometry, does not modify the protein structure, which forms a homotrimer with a ring-shaped structure. PCNA embraces the DNA and slides over the double helix anchoring different enzymes that modify DNA (helicases, polymerases, nucleases, and ligases) and proteins that regulate its replication and repair. These proteins bind by a conserved sequence called PIP (PCNA-interacting protein) to a region of the PCNA protomer close to Tyr211, whose phosphorylation affects replication fork stability as seen in cellular assays.
The work team hypothesized that phosphorylation interfered with the molecular recognition of other proteins through their PIP sequences. However, quantitative measurements of the affinity for fragments corresponding to the PIP sequences of two regulatory proteins (p21 and p15) show a minimal effect. Binding of the PIP sequences of DNA polymerase delta, and deubiquitinase USP29 is of very low affinity and the effect of phosphorylation could not be tested, although it is probably not significant either.
The results presented in this paper suggest that the possible effect is manifested only in the context of the chromatin. However, the existence of another factor, not yet identified and different from the PIP sequence motif, which may be responsible for the effect of Tyr211 phosphorylation on DNA replication, has not been ruled out.
Reference:
Ruiz-Albor A, Chaves-Arquero B, Martín-Barros I, Guerra-Castellano A, Gonzalez-Magaña A, de Opakua AI, Merino N, Ferreras-Gutiérrez M, Berra E, Díaz-Moreno I, Blanco FJ. PCNA molecular recognition of different PIP motifs: Role of Tyr211 phosphorylation. Int J Biol Macromol. 2024 Jun 14;273(Pt 2):133187. doi: 10.1016/j.ijbiomac.2024.133187.

