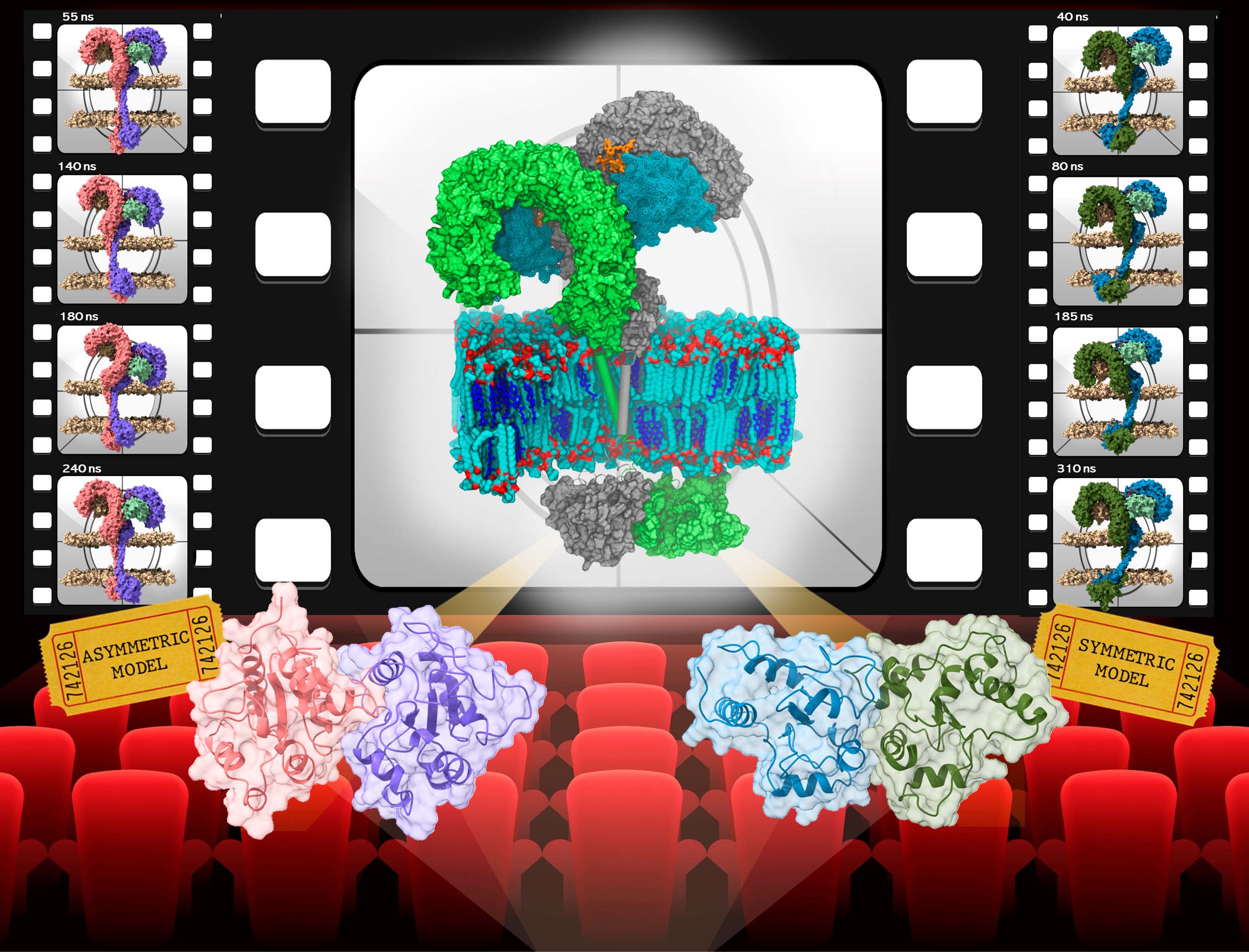
A study recently published in Chemistry a European Journal by the group of Sonsoles Martín-Santamaría at the Margarita Salas Center for Biological Research (CSIC) has provided the most realistic and complete 3D models to date of the active full Toll-like receptor 4 (TLR4) complex embedded into a realistic membrane, through a computational study combining ab initio, molecular docking, and thermodynamics calculations, pushing against the limits of all-atom molecular dynamics simulations.
The TLR4 is a membrane receptor of paramount importance as a therapeutic target as one of the main actors in innate immunity. Its assembly, upon binding of Gram-negative bacteria lipopolysaccharide (LPS), and also dependent on the membrane composition, finally triggers the immune response cascade.
Matamoros-Recio et al. give functional and structural insights into the transmembrane domain behavior in different membrane environments, the ectodomain bouncing movement, and the dimerization patterns of the intracellular domain, accounting for the active (agonist) state of the TLR4, and pointing to a signal transduction mechanism across the cell membrane.
The authors supply the hypothesis, from the thermodynamic point of view, that the TD2-symmetric and TD1-asymmetric models adopt favorable conformations of the full-length receptor in lipid rafts, upon LPS binding. From the molecular point of view, both full-length TLR4 models are suitable for binding either monomeric or dimeric MAL adaptors in the plasma membrane. Thus, it is suggested that both ways of dimerization could co-exist, and have a direct implication in the activation of distinct TLR4 pathways. The most favorable energies are observed when the extracellular domain is tilted towards the membrane, interacting with the phospholipids head groups, pointing to a signal-transduction mechanism across the cell membrane, within lipid–TLR4 interactions.
Therefore, the results concerning the effect of the membrane composition on the structure and dynamics of the TLR4 are of particular biological significance due to its role in signal transduction events. In this receptor–lipid interplay, it can be speculated that the observed dynamic bouncing motion is proper behavior for membrane receptors that can work as antennas for detecting pathogens. These observations unveil relevant molecular aspects involved in the TLR4-related innate immune pathway, opening new opportunities for advancing in the TLR4 modulation.
This paper has also been selected for “Cover feature”, which can be accessed here.
Reference: Full-Atom Model of the Agonist LPS-Bound Toll-like Receptor 4 Dimer in a Membrane Environment. Matamoros-Recio A, Franco-Gonzalez JF, Perez-Regidor L, Billod JM, Guzman-Caldentey J, Martin-Santamaria S. Chemistry. 27, 15406-25 (2021). doi: 10.1002/chem.202102995.

