Bacterial cell division in many organisms involves a constricting cytokinetic ring that is orchestrated by the tubulin-like protein FtsZ. FtsZ forms dynamic filaments close to the membrane at the site of division that were recently discovered to treadmill around the division ring, guiding septal wall synthesis.
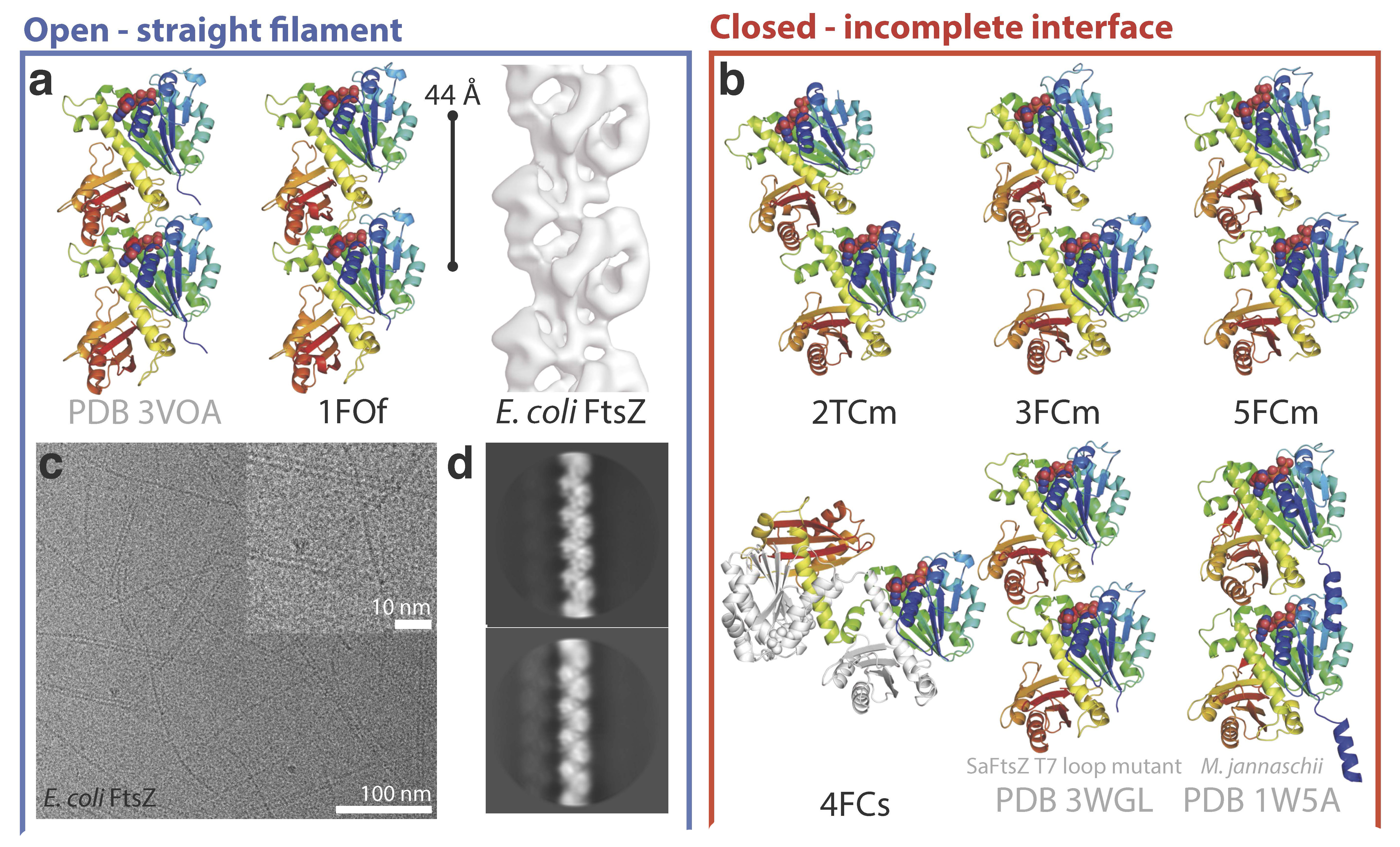
A new study published in mBIO by the groups of Jan Löwe at the MRC-LMB and José Manuel Andreu at CIB has now revealed how FtsZ can adopt two functionally distinct conformations, open and closed, using X-ray crystallographic data of Staphylococcus aureus SaFtsZ together with biochemical assays.
The FtsZ assembly switch had been biochemically demonstrated before at CIB. Non-polymerizing FtsZ mutants were designed and characterized for this work, where the authors show the structural mechanism of the switch. The open structure is found in SaFtsZ filaments formed in crystals and also in soluble filaments of Escherichia coli FtsZ as deduced by cryoEM. The closed structure is found within several crystal forms of non-polymerizing SaFtsZ and corresponds to many previous FtsZ structures from other organisms.
All together these results point out that FtsZ undergoes a polymerization-associated conformational switch. Such a switch provides explanations for both how treadmilling may occur within a single-stranded filament, and why filament assembly is cooperative, two features previously thought exclusive of multi-stranded cytomotive filaments.
Reference: A polymerisation-associated structural switch in FtsZ that enables treadmilling of model filaments. James Mark Wagstaff, Matt Tsim, María A Oliva, Alba García-Sanchez, Danguole Kureisaite-Ciziene, Jose M Andreu, Jan Löwe. mBIO (2017) 8, e00254-17

