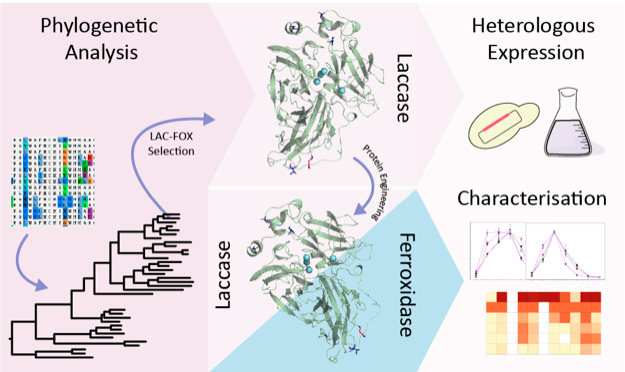
A work recently published in the Computational and Structural Biotechnology Journal by the group led by Dr. Susana Camarero at Centro de Investigaciones Biológicas Margarita Salas (CSIC) has shown the existence of two evolutionary branches within the laccase-ferroxidase family of multicopper oxidases, that correlates with the presence or absence of some structural determinants underlying their putative hybrid activity.
The multiple copper oxidase (MCO) superfamily encompasses a large variety of enzyme sequences that share a common molecular architecture and the presence of copper ions as cofactors for reducing O2 to H2O but differ in their oxidizing capabilities and physiological function. The complexity of the MCO superfamily requires structure-function and phylogenetic studies for better characterization and classification of the different MCO families (laccases, ferroxidases, ascorbate oxidases, etc.). Actually, many new emerging MCO genes are wrongly annotated as laccases, the most broadly applied MCOs (particularly those secreted by saprotrophic basidiomycete fungi) due to their ability to oxidize lignin and a collection of aromatic compounds.
Laccase-ferroxidases (LAC-FOX) are one of the reported but a still largely unexplored group of MCOs, for which dual activities are proposed. Aza and co-workers have performed a phylogenetic analysis of all LAC-FOXs from basidiomycete fungi and found two evolutionary branches that correlated with the presence or absence of some of the three acidic residues responsible for ferroxidase activity in Fet3p from S. cerevisiae. Enzymes from subgroup 1 (with two analogous acidic residues) show efficient ferroxidase activity and some laccase-like activity. Enzymes from subgroup 2 (with only one acidic residue) show insufficient or null ferroxidase activity but good laccase activity.
The latter was confirmed by the heterologous expression and characterization of the LAC-FOX from Heterobasidion annosum. The recombinant enzyme oxidized typical laccase substrates but had no ferroxidase activity. Of the mutated variants, only that holding the three acidic residues exhibited efficient Fe (II) oxidation, while partially retaining the oxidative activity of the native enzyme on aromatic compounds.
These results shed light on key catalytic residues driving ferroxidase or laccase activities in MCO enzymes while opening new scenarios for their application.
Reference: Multicopper oxidases with laccase-ferroxidase activity: Classification and study of ferroxidase activity determinants in a member from Heterobasidion annosum s. l. Pablo Aza, Gonzalo Molpeceres, Jesper Vind, Susana Camarero. Computational and Structural Biotechnology Journal 21 (2023) 1041–1053. https://doi.org/10.1016/j.csbj.2023.01.030

