A recent work published in the journal iScience by the group of Dr. Miguel Peñalva at Centro de Investigaciones Biológicas Margarita Salas (CSIC), in collaboration with Ernesto Arias’ group and the Proteomics Facility at the same center, has led to the identification and characterization of a protein complex that adapts myosin V to secretory vesicles.
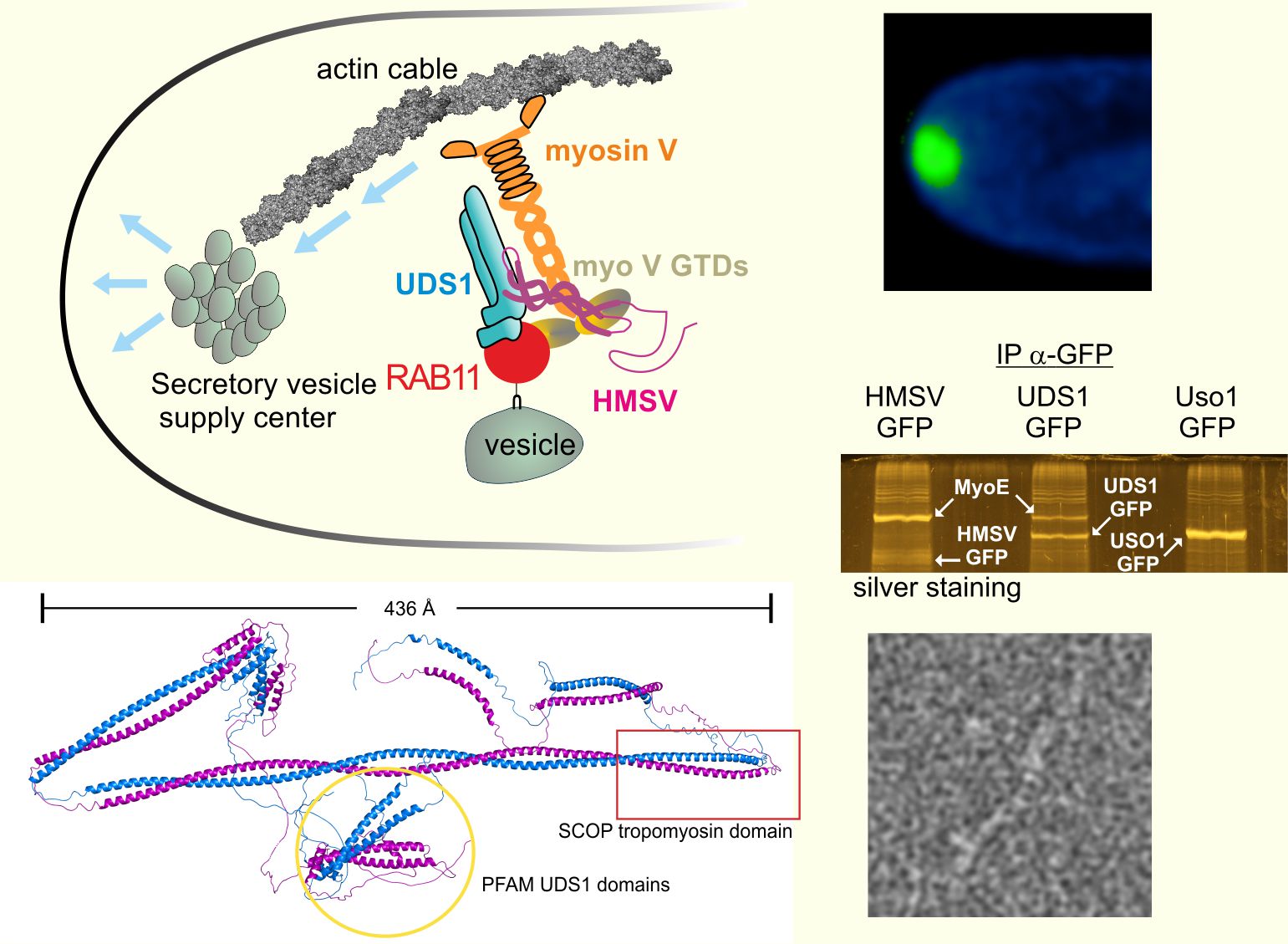
Aspergillus nidulans has long (> 100 mm) tubular cells that grow by apical extension, being ideally suited to investigate intracellular transport and cooperation between microtubule and actin-dependent motors. Secretory vesicles are transported to the tips by kinesin-1, where they are transferred to myosin V, which focuses them on a vesicle supply center located underneath the plasma membrane. Up to date, how a single type V myosin recognizes the multiplicity of cargoes that move on actin filaments is insufficiently understood.
Pinar et al. characterize the HUM complex, which adapts myosin to these secretory vesicles. The complex is formed by the protein RAB11 and two as yet uncharacterized accessory proteins that help RAB11 to recruit myosin V and are denoted UDS1 and HMSV (hook of myosin to secretory vesicles). These two proteins, showing a high content of coiled coils and unstructured regions, are strong candidates to play structural roles in the stability/assembly of the vesicle supply center, which might represent a further example of a membrane-less organelle.
Reference: The type V myosin-containing complex HUM is a RAB11 effector powering movement of secretory vesicles. Mario Pinar, Ana Alonso, Vivian de los Ríos, Ignacio Bravo-Plaza, Álvaro de la Gándara, Antonio Galindo, Ernesto Arias-Palomo, Miguel Á. Peñalva (2022) Volume 25, Issue 7, 104514. https://doi.org/10.1016/j.isci.2022.104514

