[This text originally appeared as an Editorial in the CSIC website, in Spanish]
While we are still in the midst of the coronavirus pandemic, some worrying data are increasingly announcing the proximity of another, perhaps more silent but no less threatening pandemic: the spread of "superbugs" resistant to the entire arsenal of antibiotics available today. In fact, the increasing emergence of bacterial strains resistant to certain antibiotics has been occurring for decades, but it is only in recent years that some bacterial pathogens are becoming practically pan-resistant. Moreover, it has been observed that in this pandemic period the consumption of some antibiotics has skyrocketed and, in parallel, so has the rate of resistant bacteria. This bleak outlook has prompted an alert from the World Health Organization (WHO) through its action plan against antibiotic resistance. In this regard, a few days ago the "European day for the prudent use of antibiotics" was celebrated, which is being continued in Spain with other campaigns that stress the same message: use antibiotics rationally and avoid their abuse, the main factor in the increase of resistance.
While other causes of death in the world are being combated with some success, those caused by these multidrug-resistant bacteria, and those expected in the coming years, are being faced with growing alarm and even pessimism. This is mainly because the major pharmaceutical companies have withdrawn, in recent years, a large part of their investments in new antibacterial drugs. The result is that, not without reason, the health authorities seem to have agreed to sound the alarm with the threatening numbers of deaths expected in the coming decades "if the situation continues as it is and there are no other treatments". But this analysis often does not go further than that as to whether alternative solutions are really on the horizon.
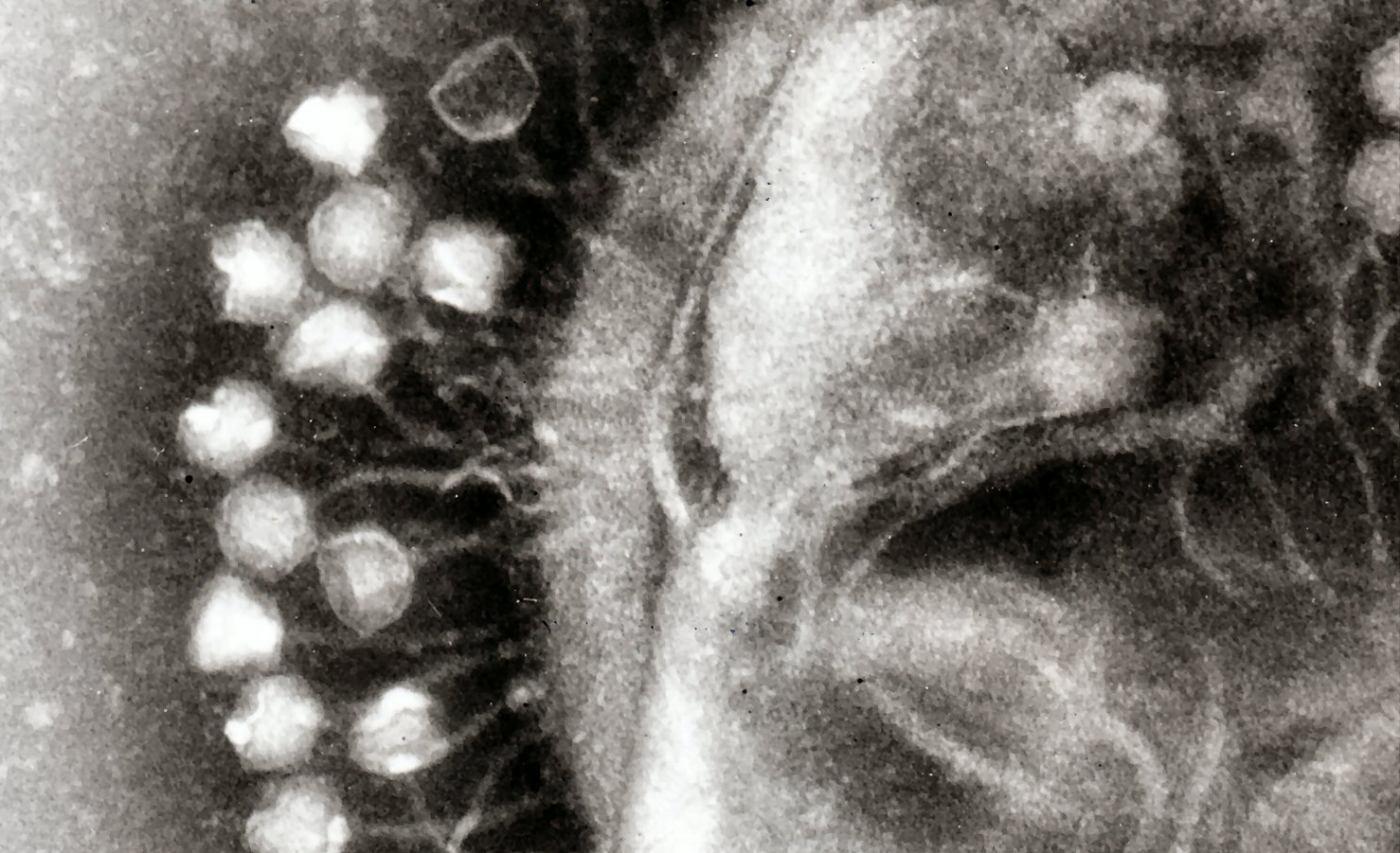
And yet, today it can be clearly stated that there are promising treatments based, for example, on the use of bacteriophages, or phages for short, known as "phage therapy". Phages, discovered just over a century ago, are viruses that exclusively infect bacteria. They are the most abundant biological entities on the planet and, moreover, the dominant predators in the biosphere, being found normally and in large numbers even alongside the microbiota that inhabit our own bodies. In general, their life cycle involves the lysis and death of the host bacteria and, for this reason, their therapeutic application to combat some infections was adopted, at least experimentally, very soon after their discovery (remember, more than a century ago). However, the discovery of penicillin and other families of antibiotics, towards the middle of the last century, relegated the use of phages in the West almost to oblivion, until the increasing appearance of resistant bacterial strains rescued the scientific interest not only of the whole phages, called virions, but also of some products encoded by phages, especially the enzymes that break the murein, the envelope that all bacteria have as a protective shell. These enzymes are called endolysins and, when used as purified proteins with bactericidal activity, they are also called "enzibiotics".
In recent years, numerous scientific articles have been published that have demonstrated the great bactericidal potential of phages and enzibiotics, not only in in vitro laboratory experiments, but on many occasions the results have been validated in animal models of infection and have even reached the stage of clinical trials in humans for some of these products with, in many cases, encouraging results. In general terms, phage therapy has several advantages over traditional antibiotics. Among them is the fact that the action of phages and enzibiotics is generally fast and specific, so that in clinical cases of bacterial infections only the pathogenic bacteria causing the disease are affected, leaving the other beneficial bacteria of the microbiota intact. Moreover, the fact that bacteria are resistant to antibiotics does not imply that they are also resistant to phages or enzibiotics; moreover, in general, there is no cross-resistance, which makes them a clear alternative for the elimination of these bacteria. Another point in favor of phage therapy is that the resistant mutants that can easily appear against both antibiotics and phages during their therapeutic administration can be effectively avoided: for treatments with whole phages, and given the great abundance and diversity of phages available in the environment, a cocktail of those most active against the specific pathogenic bacterium is usually chosen, which greatly minimizes the probability that the bacterium will have time to acquire mutations against all of them. In the case of enzibiotics, experimental practice has shown that the emergence of mutants resistant to them is very unlikely. Another obvious advantage over antibiotics, derived from the huge number of phages and their environmental ubiquity, is that obtaining and purifying them is a quick and inexpensive task. Moreover, the enormous biological diversity of phages is a virtually inexhaustible source of new antibacterial agents which, making use of new technologies in synthetic biology, may allow, in the medium term, the development of "tailor made" therapeutic molecules, perfectly adapted to the needs of each specific infectious condition.
It is important to note that in some hospitals in several European countries and in the United States, there have already been cases of authorized clinical use of phages as compassionate therapy to treat patients affected by serious infections caused by multidrug-resistant bacteria against which conventional antibiotic therapy was no longer a viable therapeutic option. Probably one of the most talked-about cases was described a couple of years ago in the journal Nature Medicine, where a 15-year-old British patient was completely cured by intravenous administration of a cocktail of three phages modified in the laboratory to effectively attack the strain of Mycobacterium abscessus that was damaging her vital organs. This is obviously not the only example: to date, many more cases of successful treatments against a variety of multidrug-resistant pathogens have been reported worldwide, some of them also in Spain. In the case of enzibiotics, about three years ago the first of these was marketed, for topical use, directed against methicillin-resistant Staphylococcus aureus bacteria. Other enzibiotics are currently in advanced clinical validation phases, which augurs that in the medium term these types of antibacterials will be available to combat infections caused by various superbugs. In fact, what is expected to be an exemplary case for the near regulation and commercialization of enzibiotics, Exebacase from the U.S. company Contrafect, will complete the third and final stage of its clinical trials for use in systemic S. aureus infections next year.
In Spain we have an important academic and even business network that places us in a good position for the adoption of these new therapies. A good example of this is the FAGOMA network, to which the authors of this article belong. As part of its activities, FAGOMA has set out to raise awareness of phage research and application, with the short-term aim of stimulating a public debate that will culminate in the regulation of this type of therapy to allow its routine application with full legal security and health guarantees. Because we think that phage therapy, with its weaknesses and difficulties, of which it is not exempt, is going to be in the near future at least one of the main solutions to fight against resistant bacteria, as an alternative or complement to antibiotics. Our society needs to have this debate now and get ahead of the wave of pandemics to come.
Pedro García. Centro de Investigaciones Biológicas Margarita Salas (CIB-CSIC), and Centro de Investigación Biomédica en Red de Enfermedades Respiratorias (CIBERES), Madrid (pgarcia@cib.csic.es).
Roberto Vázquez. Department of Biotechnology, Ghent University, Ghent, Belgium (rvazqf@gmail.com)
Pilar García. Instituto de Productos Lácteos de Asturias, IPLA-CSIC, Asturias (pgarcia@ipla.csic.es)
The three authors belong to the working group on Phage Therapy of the Spanish Network of Bacteriophages and Transducer Elements (FAGOMA).
More information (more information):
Pedro García was interviewed on Radio Ecca. The audio can be accessed here.
Listen to this report appeared on "A hombros de Gigantes" (RNE) with the participation of Pedro García.

