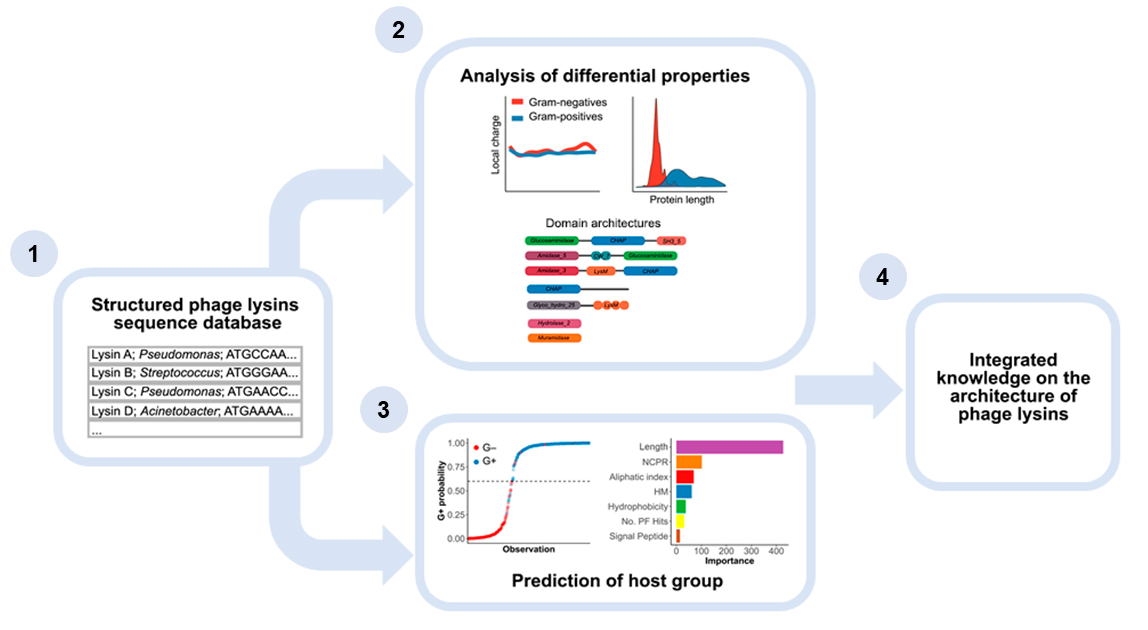
A recent article in the Journal of Virology, co-signed by Roberto Vázquez, Ernesto García, and Pedro García, from the group Host-parasite interactions in pneumococcal infections at the Centro de Investigaciones Biológicas Margarita Salas, has provided an updated analysis of the main structural characteristics of phage lysins. Furthermore, the study has made it possible to clarify the evolutionary importance of several architectural properties of lysins, particularly in the mechanism of interaction with the surface of Gram-negative bacteria.
Phage-encoded lytic enzymes, also called lysins or enzybiotics, are one of the most promising alternatives to standard antibiotics. The potential of enzybiotics as new antimicrobials to combat antibiotic-resistant bacteria arises not only from characteristics such as a lower chance of causing resistance, but also from their versatility for use in different modular protein engineering strategies. Currently, lysin-derived functional modules are being used for the design of new antimicrobials with the desired properties.
The published article provides insight into phage lysin diversity by examining a set of lysin genes. In this way, the fundamental differences between the lysins of the phages that infect bacteria with different architectures on their surface have been unraveled and, therefore, also the relevance of certain properties in terms of their specialization in the lysis of specific structures of the cell wall. Specifically, it has been observed that phage lysins that infect Gram-negative bacteria mostly contain elements similar to antimicrobial peptides (subdomains called “AMP-like”) that probably facilitate binding to the outer membrane and its disruption to allow the efficient lysis of Gram-negative hosts.
These results provide evidence to support some of the common hypotheses in the current literature ―such as the aforementioned presence of AMP-like regions, or the fact that lysins from Gram-negative infecting phages bear a single catalytic module while those from Gram-positives are multimodular―, as well as make available to the scientific community an updated database of lysin sequences for future developments.
Reference: Sequence-function relationships in phage-encoded bacterial cell wall lytic enzymes and their implications for phage-derived products design. Vázquez R*, García, E, García, P. (2021) Journal of Virology 95:e00321-21. https://doi.org/10.1128/JVI.00321-21.

