A work published in Cell Mol. Life Sci. by the team led by Dr. Susana Camarero in the Group of Biotechnology for Lignocellulosic Biomass at Centro de Investigaciones Biológicas Margarita Salas (CSIC) has described the optimization of the α-factor preproleader to improve recombinant production of fungal enzymes in Saccharomyces cerevisiae.
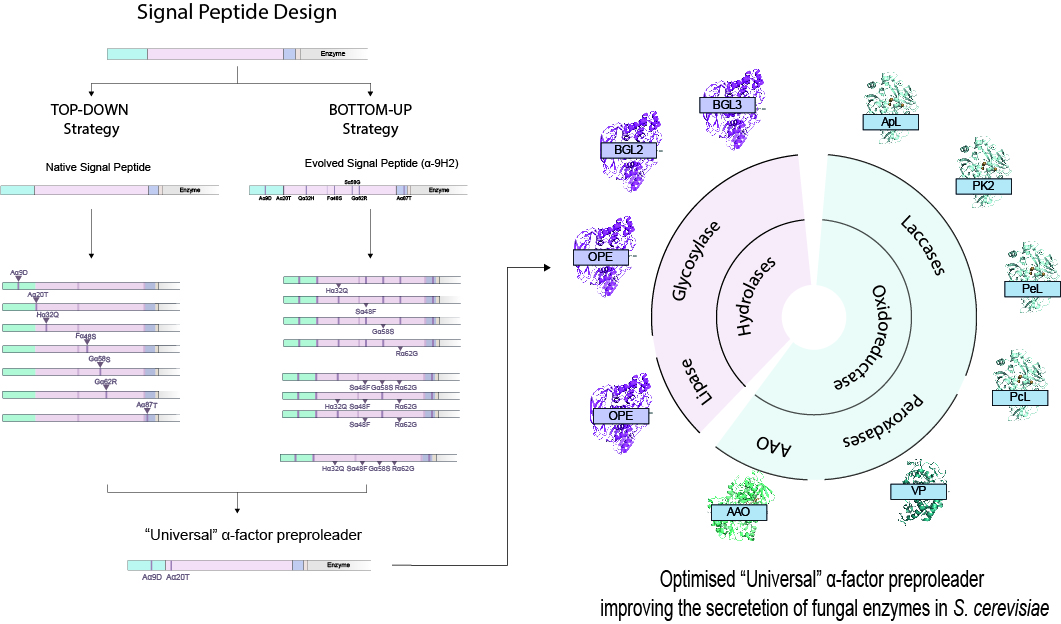
The yeast S. cerevisiae has been historically utilized as an expression system for eukaryotic proteins due to its easy manipulation, low requirements, and the ability for protein post-translational modifications. One of the best strategies to enhance secretion of the recombinant proteins is the use of yeast signal peptides which determine the secretion pathway. Among them, the preproleader sequence of the α-factor mating pheromone of S. cerevisiae has played an important role in the production of recombinant proteins from different sources in yeast. In previous studies carried out by the group, the use of this signal peptide combined with enzyme-directed evolution allowed to achieve the otherwise difficult functional expression of fungal laccases in S. cerevisiae, obtaining different evolved α-factor preproleader sequences that improve laccase secretion. However, the designing of a universal signal peptide to enhance the production of heterologous enzymes in S. cerevisiae is a pending challenge.
In this study, researchers used two parallel engineering strategies for the optimization of the α-factor preproleader to improve recombinant enzyme production in S. cerevisiae, aiming to analyze the effect of mutations accumulated in the signal sequence throughout iterations of enzyme directed evolution, or other reported mutations, and their possible epistatic interactions. Both approaches agreed on the positive synergism of four mutations contained in the final optimized leader sequence which notably enhanced the secretion of several fungal oxidoreductases and hydrolases. Additionally, they were able to suggest a guideline to further drive the heterologous production of a particular enzyme based on combinatorial saturation mutagenesis at two concrete positions of the optimized leader fused to the target protein.
Achieving sufficient levels of recombinant enzyme production in heterologous hosts is needed to conduct protein engineering research, apart from being an indispensable requisite for any desired application testing or product commercialization. Considering that S. cerevisiae is one of the most widely chosen expression systems for fungal enzymes, the results obtained in this study offer an important advancement to enhance protein secretion, providing a “universal”, optimized signal peptide to be used for this endeavor.
This work has been carried out in the frame of the European Project WoodZymes coordinated by Dr. Susana Camarero, where the role of the group of Biotechnology for Lignocellulosic Biomass is to obtain engineered fungal laccases with extremophilic properties, able to work at the highly demanding conditions of elevated pH and temperature required by the wood transforming industry. The obtained results will contribute to increasing the production levels of the engineered biocatalysts. The WoodZymes project has received funding from the Bio-Based Industries Joint Undertaking, part of the European Union’s Horizon 2020 research and innovation program, under grant agreement No 792070.
Reference: Design of an improved universal signal peptide based on the α-factor mating secretion signal for enzyme production in yeast. Aza P, Molpeceres G, de Salas F and Camarero S (2021) Cell Mol. Life Sci. https://doi.org/10.1007/s00018-021-03793-y

