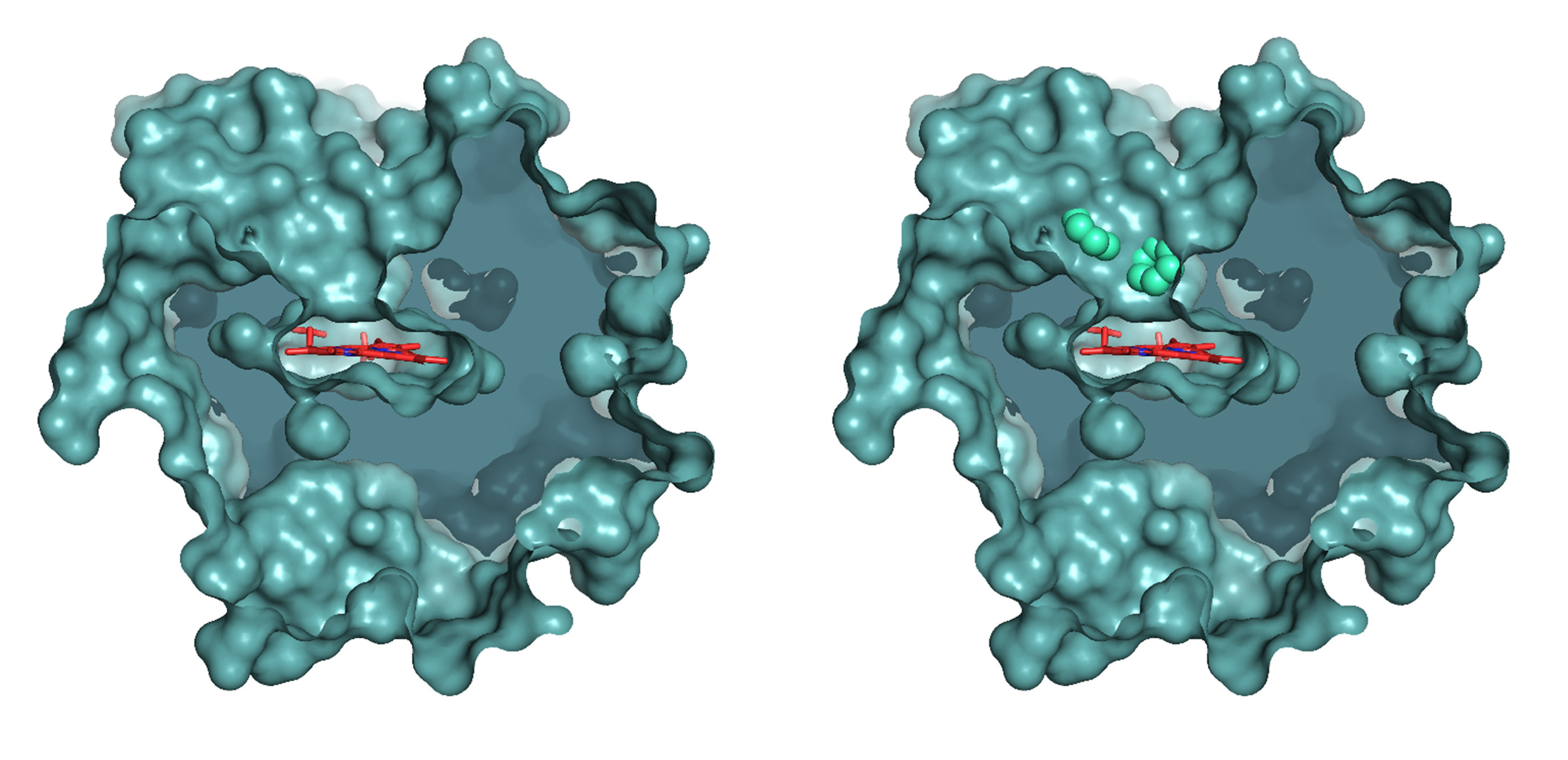
A work recently published by the group of "Biotechnology for Lignocellulosic Biomass" at Centro de Investigaciones Biológicas Margarita Salas (CIB-CSIC) in the journal ACS Catalysis presents the development of novel fungal peroxygenases, which behave as “P450s with advantages” for oxygenation biocatalysis thanks to their self-sufficient monooxygenase activity. As a result of an international collaboration, the study has also described the structural basis for their catalytic properties on different fatty acids and, besides, has shown their potential to produce compounds of high-added value at preparative scale.
Municoy M. et al. have used powerful computational tools, such as the software PELE for ligand diffusion into proteins, to simulate the behaviour of the enzymes on their substrates, being able to satisfactorily predict the oxygenation type (epoxidation versus hydroxylation) of the new peroxygenases. Computational data have been combined with the experimental analysis of the oxygenated products formed, and process scale-up. Factors related to the enzymatic active-site architecture and the double-bond distribution in the different fatty acids were suggested by the computational analyses, identified in experimental reactions, and confirmed by site-directed mutagenesis of the heme access channel. In this way, the structural determinants for the catalytic properties of the different enzymes analyzed have been unveiled.
The potential of this combined computational-experimental approach to develop new biotechnological processes for the enzymatic production of biobased chemicals was further demonstrated by the preparative regio and stereoselective epoxidation of α-linolenic acid. The product of this reaction, cis,cis-15(R),16(S)-octadeca-9,12-dienoic acid, was obtained employing wild and engineered peroxygenases, attaining 80−83% enantiomeric excess and over 99% regioselectivity. This and other monoepoxides from polyunsaturated fatty acids are nearly impossible to be obtained by chemical means. Therefore, their enzymatic synthesis, developed as a final output of the present study, represents a new and interesting reaction given the biological activity of these compounds, in addition to their application in organic chemistry as highly reactive molecules.
The above tools are being evaluated in the frame of the SusBind European project, which aims to develop new fully-biobased and environmentally-friendly adhesives for IKEA (and other companies) in substitution of formaldehyde-based ones. The biobased adhesives include epoxides of unsaturated fatty acids, which are produced using the abovementioned enzymes and vegetable oil feedstocks from the company Cargill. The SusBind project has received funding from the Bio Based Industries Joint Undertaking, part of the European Union’s Horizon 2020 research and innovation programme, under grant agreement No 792063.
The work is the result of a collaboration between the CIB-CSIC (Madrid), the IRNAS-CSIC (Seville), the Barcelona Supercomputing Center (Barcelona), the University of Dresden (Germany) and JenaBios SME (Germany).
Reference: Fatty-acid oxygenation by fungal peroxygenases: From computational simulations to preparative regio- and stereoselective epoxidation. Municoy M., González-Benjumea A., Carro J., Aranda C., Linde D., Renau-Mínguez C., Ullrich R., Hofrichter M., Guallar V., Gutiérrez A., and Martínez A.T. 2020. ACS Catal. http://dx.doi.org/10.1021/acscatal.0c03165

