A work published in the J. Med. Chem. by the group of Microtubule stabilizing agents led by Dr. Fernando Díaz at Centro de Investigaciones Biológicas Margarita Salas (CIB-CSIC), has provided structural insights for the binding of noscapine to tubulin permitting to propose a mechanism for its activity as an anticancer agent.
Noscapine is a natural alkaloid that is used as an antitussive medicine. However, it also acts as a weak, non-toxic, anticancer agent in certain models through a mechanism that is largely unknown. This work, which results from an international collaboration, shows that the cytotoxic agent 7A-O-dimethoxy-amino-noscapine (7A-aminonoscapine) binds to the colchicine site of tubulin.
Oliva et al. propose that the anticancer activity of noscapine arises from a bioactive metabolite that binds to the colchicine site of tubulin to induce mitotic arrest through a microtubule cytoskeleton-based mechanism.
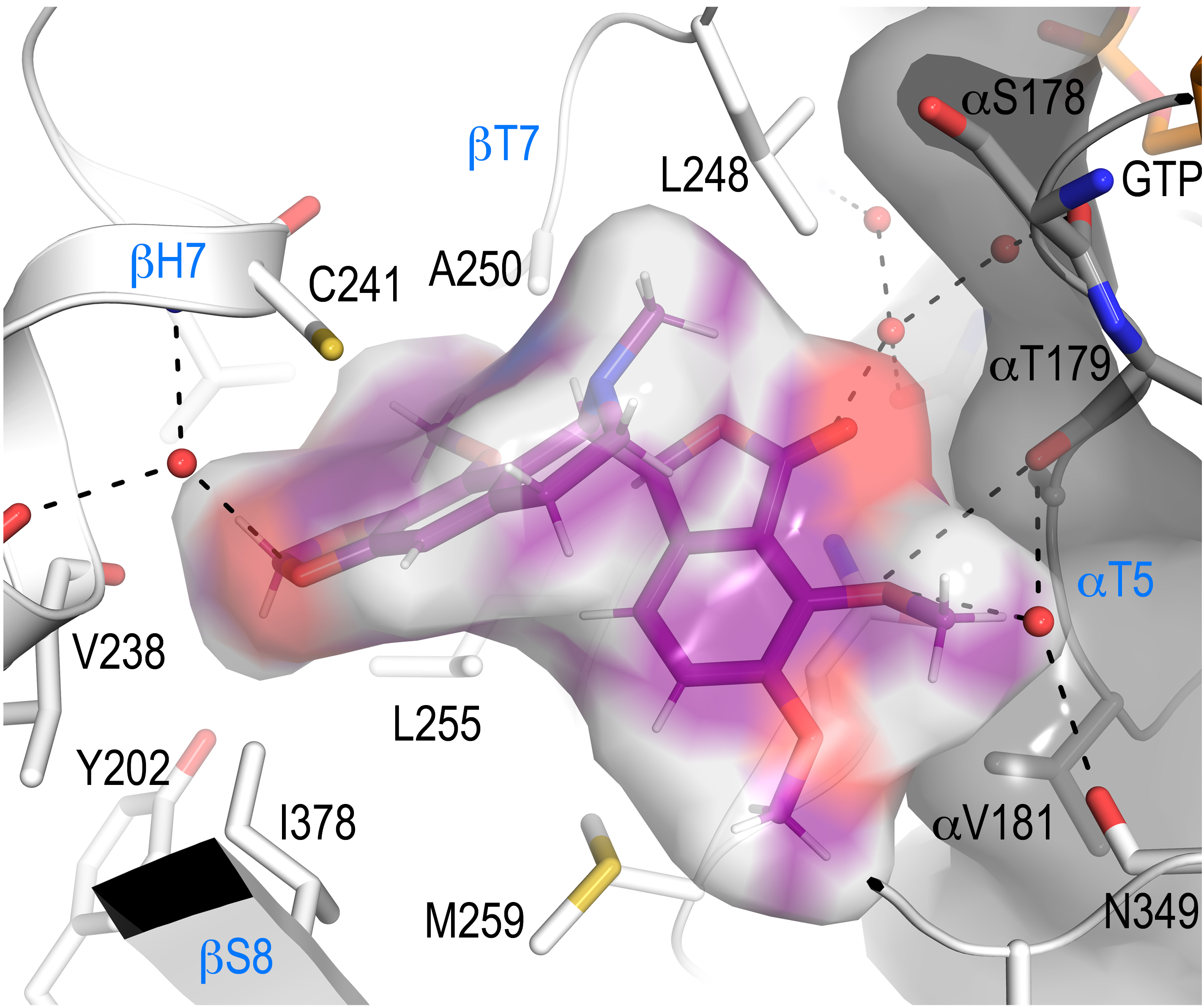
NMR-assisted docking process combining STD data and CORCEMA-ST calculations for the building of a 3D model that has been further validated through the determination of the structure of the tubulin-7A-aminonoscapine complex by macromolecular crystallography indicates that the 7A-methoxy group of noscapine prevents binding to tubulin due to a steric clash of the compound with the T5-loop of α-tubulin.
Altogether, the authors propose that noscapine’s cytotoxicity is a consequence of the oxidation of its methoxy carbon at position 7A, which leads to the formation of an active phenol. The resulting drug is then able to bind tubulin and block microtubule assembly in a colchicine-like manner.
In this regard, and oppositely to the colchicine, the other tubulin targeting compound used as immunomodulator, noscapine is not toxic, which allows its use at high doses opening a set of therapeutic possibilities for this drug.
Reference: Structural basis of noscapine activation for tubulin binding. Maria A. Oliva, Andrea E. Prota, Javier Rodríguez-Salarichs, Youssef L. Bennani, Jesús Jiménez-Barbero, Katja Bargsten, Angeles Canales, Michel O. Steinmetz, and J. Fernando Díaz (2020) J. Med. Chem. https://doi.org/10.1021/acs.jmedchem.0c00855

