A new publication by Dr. Daniel Lietha, recently incorporated to the Structural and Chemical Biology Department at Centro de Investigaciones Biológicas with a research line focused on Cell Signalling and Adhesion, has provided insights into the structure and mechanism of force sensing during cell migration. This study, published in the Proceedings of the National Academy of Sciences (PNAS), was performed in the laboratory that Dr. Lietha led at Centro Nacional de Investigaciones Oncológicas (CNIO) and is part of an international collaboration with the groups of Hermann Gaub (LMU, Germany) and Frauke Gräter (HITS, Germany).
During mesenchymal stem cell migration, cells adhere to the extracellular matrix via integrin receptors, which inside the cell are coupled to the actin cytoskeleton via a large protein complex known as focal adhesion complex. Contraction of actin stress fibers during cell migration generates tension forces in this complex which trigger important signalling cascades that control both cell migration and survival. This process is highly relevant during tumor invasion, where stiff tumor environments promote the generation of high forces in the focal adhesion complex resulting in an overactivation of migration signals that contribute to high tumor invasiveness and metastasis. Focal adhesion kinase (FAK) is a key signalling component in focal adhesions that is known to be activated upon force generation in focal adhesions, and high FAK signalling has been associated to tumor invasion and metastasis. However, the molecular basis for mechano-activation of focal adhesion signals or whether indeed FAK is the mechano-sensing molecule or it is activated downstream remained unknown.
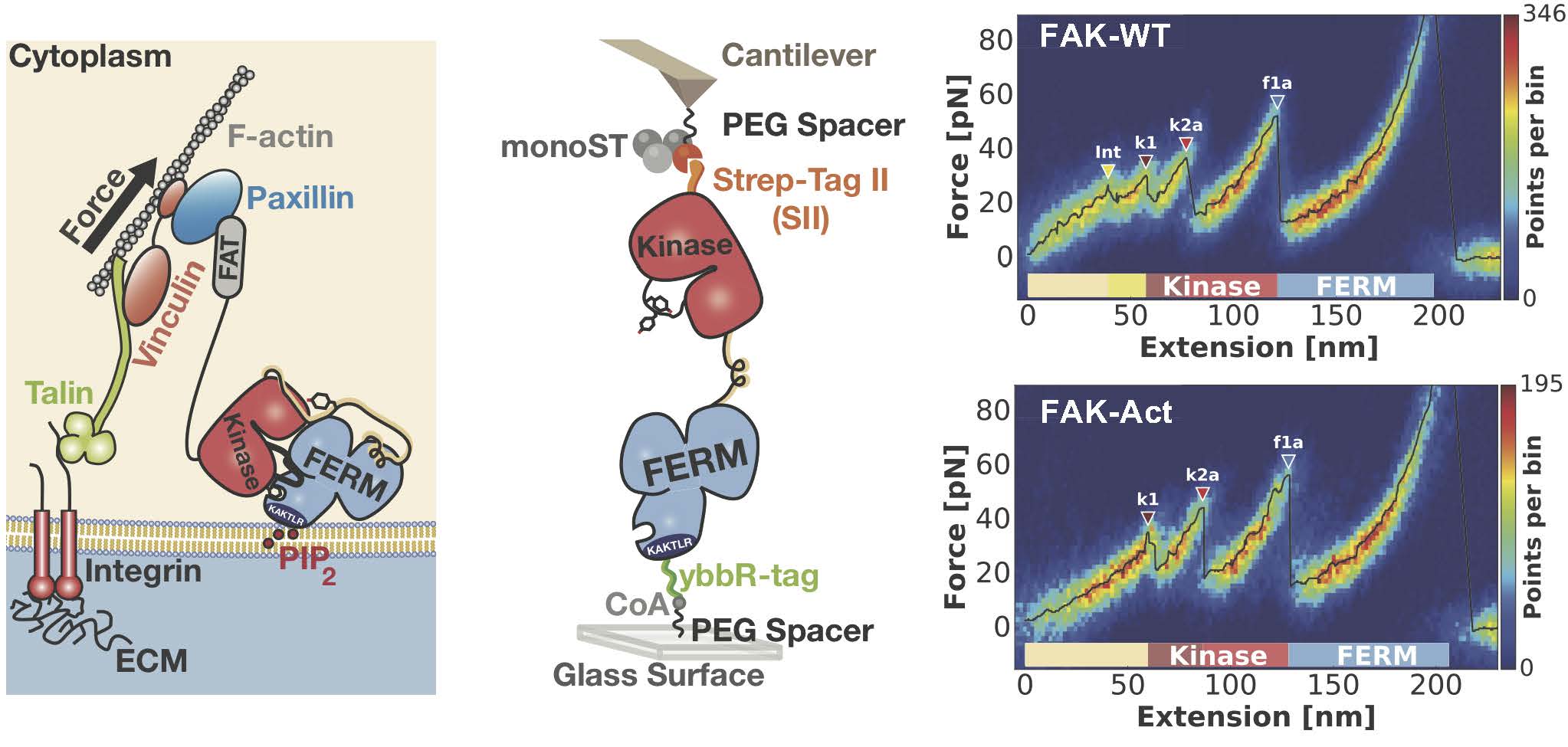
Bauer et al. investigated the mechanical properties of FAK to provide an answer to this question by studying if forces applied to FAK molecules as occurring in the focal adhesion complex result in FAK activation. Via protein engineering the authors equipped FAK molecules with suitable tags that enable attachment to an atomic force microscope (AFM) in a native geometry (Figure, middle panel). Single molecule measurements to record force-extension profiles with piconewton and nanometer precision have been obtained with a highly specialized equipment developed in the Gaub group. By overlaying hundreds of these single molecule profiles into a heat map, recurring features in the force landscape of the FAK molecule can be detected (Figure, right panel). Whereas at higher forces reproducible unfolding events of various substructures of FAK can be observed, authors also detect a low piconewton force peak prior to protein unfolding, which via comparison to a permanently activated form of FAK, can be assigned to conformational activation of FAK. The force and extension of this event fits well with the expected scenario occurring in the focal adhesion complex.
Moreover, to obtain an atomic view of FAK mechanoactivation, this process was simulated via force-probe molecular dynamics simulations by the Gräter group. The simulations are in good agreement with experimental observations and provide detailed mechanistic insights into force-induced activation of FAK.
Reference: Structural and mechanistic insights into mechanoactivation of Focal Adhesion Kinase. Magnus Sebastian Bauer, Fabian Baumann, Csaba Daday, Pilar Redondo, Ellis Durner, Markus Andreas Jobst, Lukas Frederik Milles, Davide Mercadante, Diana Angela Pippig, Hermann Eduard Gaub, Frauke Gräter, and Daniel Lietha. PNAS (2019). DOI: https://www.pnas.org/cgi/doi/10.1073/pnas.1820567116

