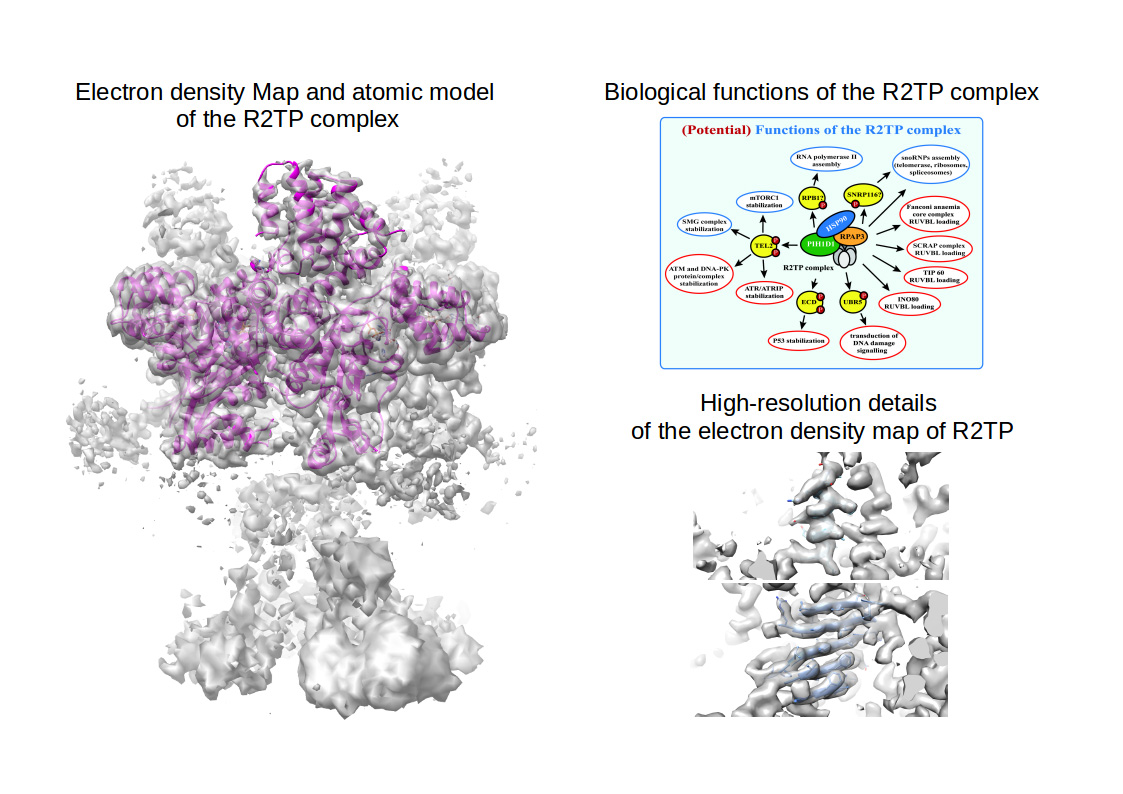
An international effort with the prominent participation of Centro de Investigaciones Biológicas, led by Dr. Óscar Llorca (staff CIB researcher until 2017), has recently published in Nature Communications the near-atomic resolution structure of the R2TP complex, an assembly machinery involved in many biological processes related to cell survival, solved by cryo-electron microscopy at 3.6 Å resolution.
R2TP is a co-chaperon that acts together with the chaperone HSP90 to supervise and guide the assembly of large biological machineries (i.e. protein complexes) that are essential for key aspects of cellular life, such as mTOR (that controls cellular growth), RNA Polymerase II (responsible for transcribing DNA into mRNA) and PI3Ks (acting in the response to DNA damage and cancer progression).
The article by Martino et al. helps to understand the molecular details how the R2TP complex functions. Indeed, Martino and colleagues have discovered that the R2TP complex acts as a central scaffold that recruits the chaperon HSP90 and all the other proteins required for maturation of mTOR, RNA Polymerase II and PI3Ks. In this context, R2TP resembles the chassis of an ambulance: a skeleton over which cells build up a machine with all the components and tools necessary to rescue and mature other molecular machineries that are essential for cell survival. Additionally, the structure of the R2TP co-chaperone complex solved by Cryo-EM at the Electron Microscopy Facility at CIB gives important details at atomic resolution which might help for designing drugs targeting diseases such as cancer and diabetes, and cellular processes such as ageing and, moreover, makes this work key as the first step towards understanding how cells produce and regulate protein complexes that are essential for their own survival.
Cryo-EM has been on the spotlight since it was elected as Technique of the year 2016 by the major scientific journals and, more recently, Jacques Dubochet, Joachim Frank, and Richard Henderson -three pioneers in the field of Cryo-EM- have been awarded with the Nobel Prize for Chemistry 2017. This technique has the advantage of allowing to investigate biological machineries in atomic detail during their functioning state and therefore to really understand how cellular processes occur at molecular level.
The structure of the R2TP is among the first high-resolution structures solved by cryo-EM in Spain and this places CIB as one of the leading institutions in the field of cryo-EM and structural biology in Spain. This work has been possible thanks to the assistance of the Electron Microscopy Facility at CIB, which implemented an efficient sample preparation work flow specific for high resolution cryo-EM.
Reference: RPAP3 provides a flexible scaffold for coupling HSP90 to the human R2TP co-chaperone complex. Fabrizio Martino, Mohinder Pal, Hugo Muñoz-Hernández, Carlos F. Rodríguez, Rafael Núñez-Ramírez, David Gil-Carton, Gianluca Degliesposti, J. Mark Skehel, S. Mark Roe, Chrisostomos Prodromou, Laurence H. Pearl & Oscar Llorca (2018) Nature Communications. doi:10.1038/s41467-018-03942-1
["Biological functions of the R2TP panel": von Morgen P, Hořejší Z, Macurek L. Substrate recognition and function of the R2TP complex in response to cellular stress. Front Genet. 2015 Feb 25;6:69. doi: 10.3389/fgene.2015.00069. eCollection 2015]

