Nonsense-mediated mRNA decay (NMD) is a quality control mechanism used by cells to remove aberrant mRNAs that originate from germline mutations in many genetic disorders, as well as errors during transcription. Importantly, it has been recently discovered that NMD plays a more general role in the regulation of gene expression but controlling the decay of a subset of mRNAs, and it has a crucial role during stem cell differentiation. Despite its relevance, the detailed molecular mechanism is still not fully understood.
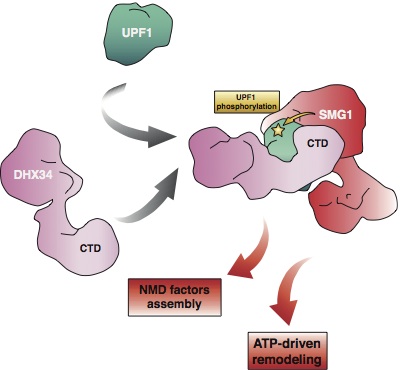
The decision to degrade or not an mRNA by the NMD pathway is decided by a set of proteins factors, mostly the UPF1, UPF2 and UPF3 proteins. NMD on a target mRNA is only trigger after UPF1 is phosphorylated by a specific kinase, named SMG1.
Some open questions in this field are how the NMD factors are specifically loaded on the target mRNA and how UPF1 phosphorylation is regulated.
In two different papers, the group of Oscar Llorca contributes to improve our understanding of this pathway for mRNA degradation.
Lopez-Perrote et al. shows in Nucleic Acids Research a mechanism by which UPF2 can be specifically loaded on the stalled ribosome by interaction with the eukaryotic release factor eRF3. Melero et al. publication in Nature Comm. on the other hand revealed some of the molecular and structural basis regulating UPF1 phosphorylation, but showing that a recently discover helicase (DHX34) functions as a scaffold that binds the substrate (UPF1) and the kinase (SMG1) to promote UPF1 phosphorylation by SMG1.

